A high-throughput assay for the measurement of Ca2+-oscillations and insulin release from uniformly sized β-cell spheroids.
A high-throughput assay for the measurement of Ca2+-oscillations and insulin release from uniformly sized β-cell spheroids.
Davidson, P.; Robben, S.; Rodrigues Ribeiro, R. S.; Voets, T.
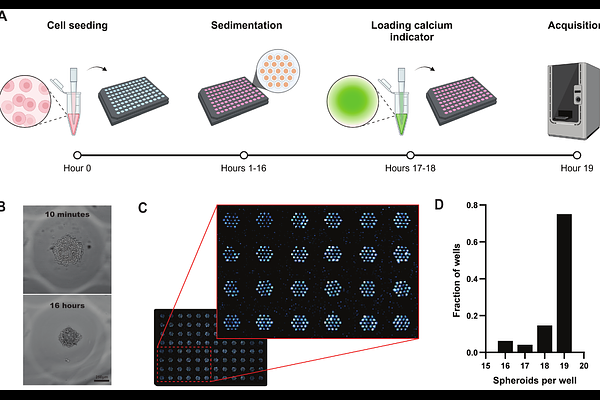
AbstractDiabetes mellitus is a rapidly growing global health challenge, necessitating the development of more effective anti-diabetic therapies, including drugs that improve insulin release from pancreatic {beta}-cells. Traditional high-throughput screening methods typically rely on 2D {beta}-cell cultures, but such cultures do not mimic the 3D organisation and cell-to-cell communication of {beta}-cells in pancreatic islets of Langerhans. Existing 3D {beta}-cell culture models are hindered by high costs, technical complexity, and limited compatibility with high-throughput screening platforms. In this work, we developed an approach for generating 19 homogeneously shaped pancreatic {beta}-cell spheroids in each well of a 96-well plate, using micropatterned polyethylene glycol (PEG)-based hydrogels and MIN6 insulinoma cells. The uniform shape and positioning of the individual spheroids enabled the simultaneous, real-time imaging of Ca2+ signals in up to 1824 independent spheroids in response to glucose and various test compounds. Using this approach, we show that increasing glucose causes concentration-dependent Ca2+ oscillations in individual spheroids, that these Ca2+ oscillations are sensitive to modulators of ATP-sensitive K+ channels, and that the frequency of Ca2+ oscillations correlate with insulin secretion. Finally, we demonstrate that the neurosteroid pregnenolone sulphate, an agonist of the cation channel TRPM3, increases the frequency of glucose-induced Ca2+ oscillations and enhances insulin release; the TRPM3 antagonist isosakuranetin inhibited these responses. In conclusion, we established a cost-effective and scalable 3D {beta}-cell platform for high-throughput screening of insulin release-modifying compounds, with potential applications in drug development and personalized medicine for the management of diabetes mellitus.