Direct observation of single intrinsically disordered proteins in solution
Direct observation of single intrinsically disordered proteins in solution
Zargarbashi, S.; Dominguez, C.; Peters, M.; Yousefi, A.; Munday, S.; Wang, Y.; Chatterjee, S.; Hudson, A.; Gordon, R.; Mellor, C. J.; Xu, L.; Rahmani, M.; Ying, C.
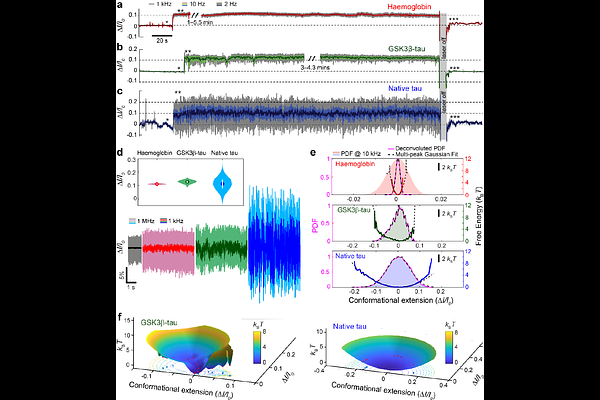
AbstractIntrinsically disordered proteins (IDPs) and proteins with intrinsically disordered regions (IDRs) are integral to many biological processes, including neurotransmitter regulation, microtubule regulation, and transcription. IDPs are heterogeneous, existing in a conformational ensemble of various interconnected states without a definitive tertiary structure. The high dynamicity of IDPs limits ensemble protein characterisation techniques from capturing their properties, and measurements at the single-molecule level are hampered by the necessity to label the protein or modify its microenvironment, affecting their biophysics. Consequently, our understanding of IDPs is limited, translating to a lack of knowledge of their roles in related diseases, including Alzheimer\'s disease, Parkinson\'s disease, and various cancers. This work presents the first experimental observation of unmodified IDP conformational dynamics in vitro, at the single-molecule level in real time, achieved by trapping individual IDPs in a nanoscale volume using aperture-based plasmonic nanotweezers. Our results reveal that IDPs exhibit significantly larger conformational variation in solution compared to globular proteins of similar size, as expected, enabling clear differentiation between the two categories. We demonstrate that phosphorylation of native tau-441 by glycogen synthase kinase 3-beta (GSK3{beta}-tau) induces compaction and reduced conformational dynamics. Furthermore, observation of the binding of an IDR (N-terminal region of Sam68, which is Src-associated protein in mitosis of 68 kDa) to G8.5 RNA presented a disorder-to-order transition. The capability of aperture-based plasmonic nanotweezers to monitor the dynamic behaviours of single, unmodified IDPs provides a powerful approach to advance our understanding of their elusive behaviours and further decode their roles in associated diseases.