Structure of the human K2P13.1(THIK-1) channel reveals a novel hydrophilic pore restriction and lipid cofactor site
Structure of the human K2P13.1(THIK-1) channel reveals a novel hydrophilic pore restriction and lipid cofactor site
Roy-Chowdhury, S.; Jang, S.; Abderemane-Ali, F.; Naughton, F.; Grabe, M.; Minor, D. L.
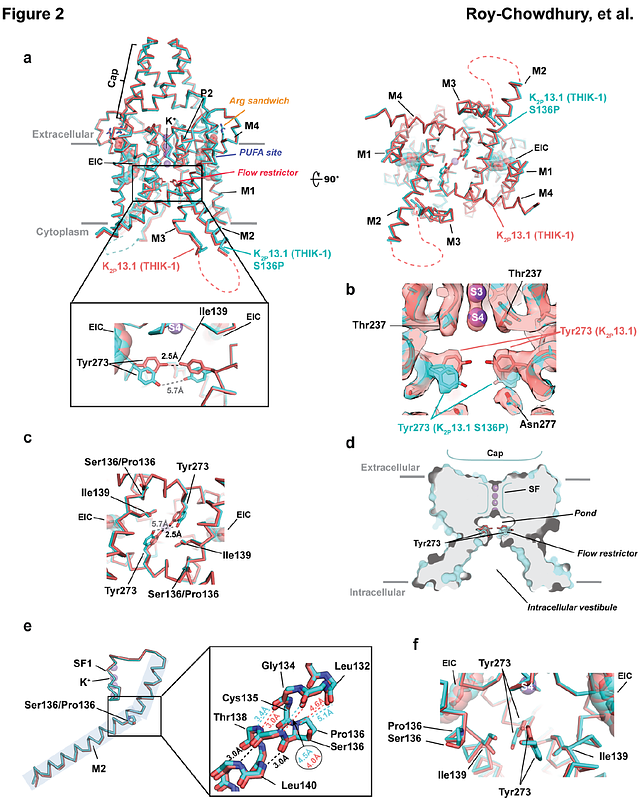
AbstractThe halothane-inhibited K2P leak potassium channel K2P13.1 (THIK-1)1-3 is found in diverse cells1,4 including neurons1,5 and microglia6-8 where it affects surveillance6, synaptic pruning7, phagocytosis7, and inflammasome-mediated interleukin-1{beta} release6,8,9. As with many K2Ps1,5,10-14 and other voltage gated ion channel (VGIC) superfamily members3,15,16, polyunsaturated fatty acid (PUFA) lipids modulate K2P13.1 (THIK 1)1,5,14,17 via a poorly understood mechanism. Here, we present cryo-electronmicroscopy (cryo-EM) structures of human K2P13.1 (THIK-1) and mutants in lipid nanodiscs and detergent. These reveal that, unlike other K2Ps13,18-24, K2P13.1 (THIK-1) has a two chamber aqueous inner cavity obstructed by the M4 transmembrane helix tyrosine (Tyr273, the flow restrictor). This hydrophilic barrier can be opened by an activatory mutation, S136P25, at natural break in the M2 transmembrane helix and by intrinsic channel dynamics. The structures also reveal a buried lipid in the P1/M4 intersubunit interface at a location, the PUFA site, that coincides with the TREK subfamily K2P modulator pocket for small molecule agonists18,26,27. This overlap, together with the effects of mutation on K2P13.1 (THIK-1) PUFA responses, indicates that the PUFA site lipids are K2P13.1 (THIK-1) cofactors. Comparison with the PUFA responsive VGIC Kv7.1 (KCNQ1)28-31 reveals a shared role for the equivalent pore domain intersubunit interface in lipid modulation, providing a framework for dissecting the effects of PUFAs on the VGIC superfamily. Our findings reveal the unique architecture underlying K2P13.1 (THIK-1) function, highlight the importance of the P1/M4 interface in control of K2Ps by both natural and synthetic agents, and should aid development of THIK subfamily modulators for diseases such as neuroinflammation6,32 and autism6.


