CHIP protects lysosomes from CLN4 mutant-induced membrane damages
CHIP protects lysosomes from CLN4 mutant-induced membrane damages
Lee, J.; Zou, J.; Mazli, W. N. A. B.; Chin, N.; Jarnik, M.; Saidi, L.; Xu, Y.; Replogle, J.; Ward, M.; Bonifacino, J.; Zheng, W.; Hao, L.; Ye, Y.
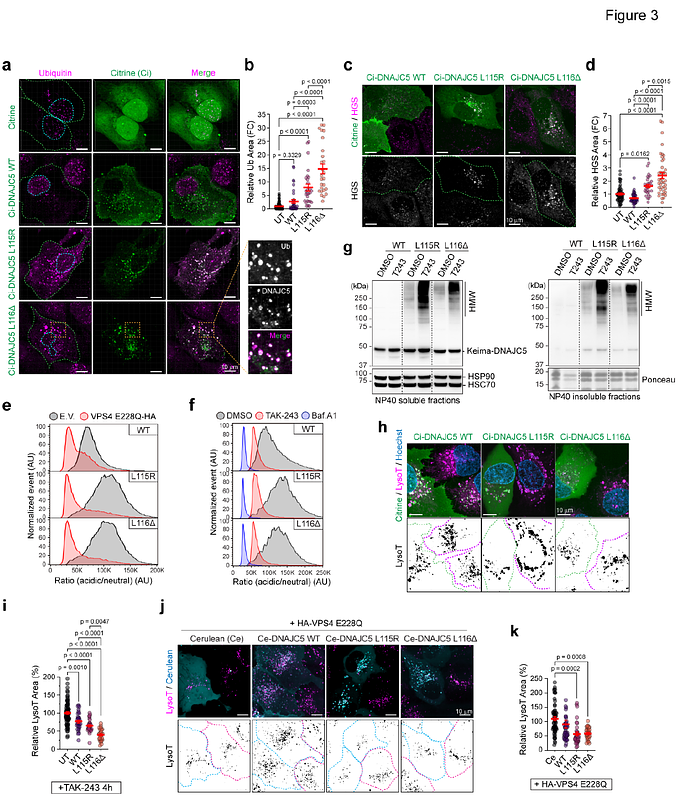
AbstractUnderstanding how cells mitigate lysosomal damage is critical for unraveling pathogenic mechanisms of lysosome-related diseases. Here we use organelle-specific proteomics in iPSC-derived neurons (i3Neuron) and an in vitro lysosome-damaging assay to demonstrate that lysosome damage, caused by the aggregation of Ceroid Lipofuscinosis Neuronal 4 (CLN4)-linked DNAJC5 mutants on lysosomal membranes, serves as a critical pathogenic linchpin in CLN4-associated neurodegeneration. Intriguingly, in non-neuronal cells, a ubiquitin-dependent microautophagy mechanism downregulates CLN4 aggregates to counteract CLN4-associated lysotoxicity. Genome-wide CRISPR screens identify the ubiquitin ligase CHIP as a central microautophagy regulator that confers ubiquitin-dependent lysosome protection. Importantly, CHIP\'s lysosome protection function is transferrable, as ectopic CHIP improves lysosomal function in CLN4 i3Neurons, and effectively alleviates lipofuscin accumulation and neurodegeneration in a Drosophila CLN4 disease model. Our study establishes CHIP-mediated microautophagy as a key organelle damage guardian that preserves lysosome integrity, offering new insights into therapeutic development for CLN4 and other lysosome-related neurodegenerative diseases.