Proinflammatory cytokine-induced alpha-cell impairment in human islet microtissues is partially restored by dual incretin receptor agonism
Proinflammatory cytokine-induced alpha-cell impairment in human islet microtissues is partially restored by dual incretin receptor agonism
Henriksen, K.; Rufer, C.; Title, A. C.; Jawurek, S.; Hartmann, B.; Holst, J. J.; Knop, F. K.; Yesildag, B.; Storling, J.
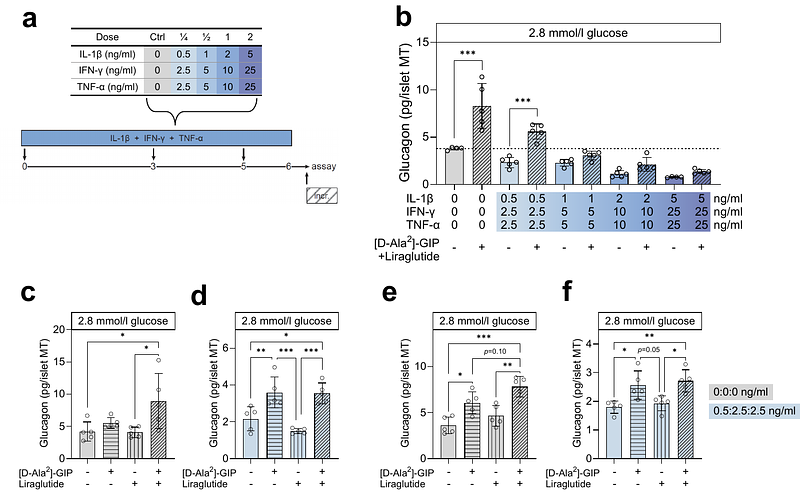
AbstractAims/hypothesis: In type 1 diabetes, the counterregulatory glucagon response to low plasma glucose is impaired. The resulting increased risk of hypoglycaemia necessitates novel strategies to ameliorate alpha-cell impairment. Here, we aimed to establish an in vitro model of alpha-cell impairment in type 1 diabetes using human islet microtissues (MTs) exposed to proinflammatory cytokines. Additionally, we investigated the therapeutic potential of incretin receptor agonists in improving alpha-cell responses to low glucose. Methods: Human islet MTs were exposed to proinflammatory cytokines (IL-1{beta}, IFN-{gamma}, and TNF-) for 1 day (short-term) and 6 days (long-term). Alpha-cell function was assessed by sequential glucose-dependent secretion assays at 2.8 and 16.7 mmol/l glucose, followed by glucagon measurements. Additional evaluations included ATP content, caspase-3/7 activity, chemokine secretion, and expression of islet transcription factors and hormones. The effects of incretin receptor agonist treatment (glucose-dependent insulinotropic polypeptide (GIP) analogue [D-Ala2]-GIP {+/-} liraglutide) alongside or after cytokine exposure were also investigated, focusing on low glucose-dependent glucagon secretion. Results: Short-term cytokine exposure increased glucagon secretion at both 2.8 and 16.7 mmol/l glucose. In contrast, long-term cytokine exposure caused dose-dependent suppression of glucagon secretion at 2.8 mmol/l glucose, resembling a type 1 diabetes phenotype. Long-term cytokine exposure also diminished somatostatin secretion, reduced ATP content, increased caspase 3/7 activity, and decreased islet transcription factor and hormone expression. Despite cytokine-induced impairment, alpha cells partially retained secretory capacity to L-arginine stimulation. Treatment with incretin receptor agonists during long-term cytokine exposure did not prevent alpha-cell impairment. However, acute treatment with [D-Ala2]-GIP {+/-} liraglutide or the single-molecule dual agonist tirzepatide partially restored glucagon secretion at low glucose. Conclusions/interpretation: Long-term cytokine exposure of human islet MTs impaired glucagon secretion to low glucose, creating a type 1 diabetes alpha-cell phenotype. This cytokine-induced alpha-cell impairment was partially restored by [D-Ala2]-GIP {+/-} liraglutide and tirzepatide, respectively.


