Connexin50 hemichannels are opened by CO2: implications for lens physiology
Connexin50 hemichannels are opened by CO2: implications for lens physiology
Lovatt, A.; Wijayapala, A.; Mui, M.; Butler, J.; Dale, N.
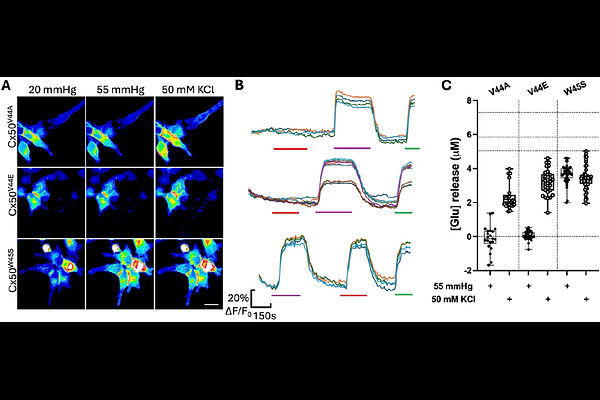
AbstractConnexin50 (Cx50) is expressed in lens fibre cells. As mutations of Cx50 cause cataracts its physiological role in the lens must be important. We have used recent cryoEM structures of Cx50 and the predictive power of alphafold3 to identify the presence of a carbamylation motif, originally described in Cx26, that suggests that Cx50 might be CO2 sensitive. By expressing the naturally truncated version of Cx50 in HeLa cells and utilising coexpression of genetically encoded sensors iGluSnFr or eLACCO1.1, we have demonstrated the CO2 sensitivity of Cx50 hemichannels, and their permeability to lactate and glutamate. Importantly, the properties of Cx50 predict that it will be partially open at the levels of PCO2 characteristic of aqueous humour. By mutating the two key residues of the carbamylation motif, K105 and K140, we have shown that the motif is required for CO2 sensitivity. Mutations of the residue V44 cause cataracts and these mutations abolish the CO2 sensitivity of Cx50. We hypothesize that CO2-dependent gating of Cx50, and consequent alterations in transmembrane Na+ fluxes, might provide homeostatic control of the microcirculation system that is critical for lens health.