Decoupling shear stress and pressure effects in the biomechanics of autosomal dominant polycystic kidney disease using a perfused kidney-on-chip.
Decoupling shear stress and pressure effects in the biomechanics of autosomal dominant polycystic kidney disease using a perfused kidney-on-chip.
Lapin, B.; Gropplero, G.; Vandensteen, J.; Mazloum, M.; Bienaime, F.; Descroix, S.; Coscoy, S.
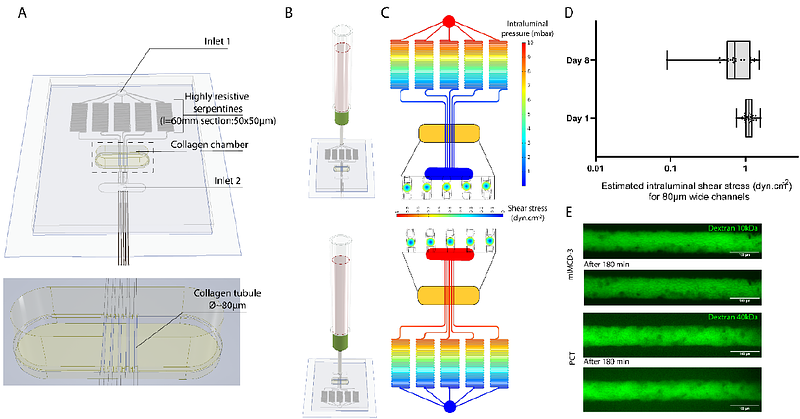
AbstractKidney tubular cells are submitted to two distinct mechanical forces generated by the urine flow: shear stress and hydrostatic pressure. In addition, the mechanical properties of the surrounding extracellular matrix modulate tubule deformation under constraints. These mechanical factors likely play a role in the pathophysiology of kidney diseases as exemplified by autosomal dominant polycystic kidney disease, in which pressure, flow and matrix stiffness have been proposed to modulate the cystic dilation of tubules with PKD1 mutations. The lack of in vitro systems recapitulating the mechanical environment of kidney tubules impedes our ability to dissect the role of these mechanical factors. Here we describe a perfused kidney-on-chip with tunable extracellular matrix mechanical properties and hydrodynamic constraints, that allows a decoupling of shear stress and flow. We used this system to dissect how these mechanical cues affect Pkd1-/- tubule dilation. Our results show two distinct mechanisms leading to tubular dilation. For PCT cells (proximal tubule), overproliferation mechanically leads to tubular dilation, regardless of the mechanical context. For mIMCD-3 cells (collecting duct), tube dilation is associated with a squamous cell morphology but not with overproliferation and is highly sensitive to extracellular matrix properties and hydrodynamic constraints. Surprisingly, flow alone suppressed Pkd1-/- mIMCD-3 tubule dilation observed in static conditions, while the addition of luminal pressure restored it. Our in vitro model emulating nephron geometrical and mechanical organization sheds light on the roles of mechanical constraints in ADPKD and demonstrates the importance of controlling intraluminal pressure in kidney tubule models.