Heterogeneous Cardiac- and Neural Crest-Derived Aortic Smooth Muscle Cells have Similar Transcriptional Changes after TGFβ Signaling Disruption
Heterogeneous Cardiac- and Neural Crest-Derived Aortic Smooth Muscle Cells have Similar Transcriptional Changes after TGFβ Signaling Disruption
Ren, P.; Jiang, B.; Hassab, A.; Li, G.; Li, W.; Assi, R.; Tellides, G.
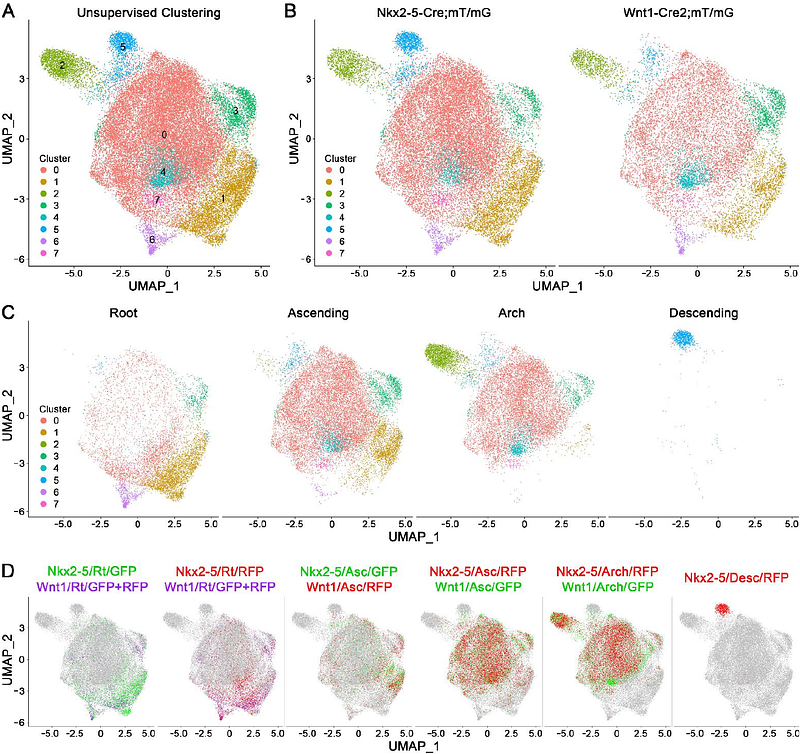
AbstractSmooth muscle cells (SMCs) of cardiac and neural crest origin contribute to the developing proximal aorta and are linked to disease propensity in adults. We analyzed single-cell transcriptomes of SMCs from mature thoracic aortas in mice to determine basal states and changes after disrupting transforming growth factor-{beta} (TGF{beta}) signaling necessary for aortic homeostasis. A minority of Myh11 lineage-marked SMCs differentially expressed genes suggestive of embryological origin. Additional analyses in Nkx2-5 and Wnt1 lineage-marked SMCs derived from cardiac and neural crest progenitors, respectively, showed both lineages contributed to a major common cluster and each lineage to a minor distinct cluster. Common cluster SMCs extended from root to arch, cardiac subset cluster SMCs from root to mid-ascending, while neural crest subset cluster SMCs were restricted to the arch. The neural crest subset cluster had greater expression of a subgroup of TGF{beta}-dependent genes suggesting specific responsiveness or skewed extracellular matrix synthesis. Nonetheless, deletion of TGF{beta} receptors in SMCs resulted in similar transcriptional changes among all clusters, primarily decreased extracellular matrix molecules and modulators of TGF{beta} signaling. Many embryological markers of murine aortic SMCs were not confirmed in adult human aortas. We conclude: (i) there are multiple subtypes of cardiac- and neural crest-derived SMCs with shared or distinctive transcriptional profiles, (ii) neural crest subset SMCs with increased expression of certain TGF{beta}-inducible genes are not spatially linked to the aortic root predisposed to aneurysms from aberrant TGF{beta} signaling, and (iii) loss of TGF{beta} responses after receptor deletion is uniform among SMCs of different embryological origins.


