Saturation kinetics and specificity of transporters for L-arginine and asymmetric dimethylarginine (ADMA) at the blood-brain and blood-CSF barriers.
Saturation kinetics and specificity of transporters for L-arginine and asymmetric dimethylarginine (ADMA) at the blood-brain and blood-CSF barriers.
Fidanboylu, M.; Thomas, S. A.
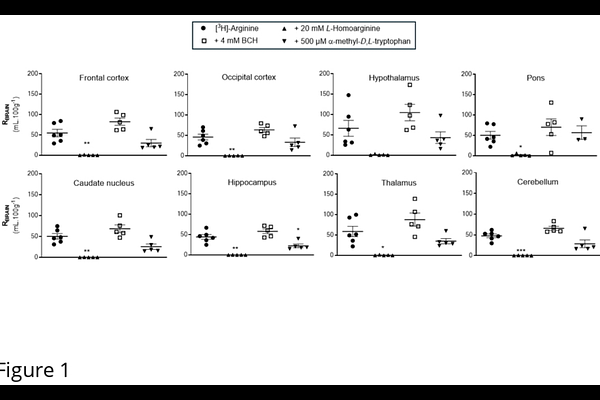
AbstractNitric oxide synthases (NOS) synthesize nitric oxide (NO) from L-arginine in endothelial and neuronal cells. Asymmetric dimethylarginine (ADMA) is a homologue of arginine and an endogenous inhibitor of NOS. As NO is a critical signalling molecule and influences physiological pathways in health and disease, the transfer of arginine and ADMA across the blood-CNS barriers is of interest. Our research group have previously demonstrated the presence of saturable transporters for [3H]-L-arginine and [3H]-ADMA at the blood-brain and blood-CSF barriers using in vitro and in situ methods. In this study, we determine the identity and kinetic characteristics of these transporters by means of the in situ brain/choroid plexus perfusion technique in anaesthetised mice. Results indicated that [3H]-arginine and [3H]-ADMA could be transported across blood-brain and blood-CSF barriers by the cationic amino acid transporter, system-y+. In contrast to the results obtained with arginine where transport was predominately by a single transport system (system-y+), ADMA delivery to the CNS was more complex and involved multiple transport systems (system y+, B0,+, y+L and b0,+) suggesting its concentration is tightly regulated. System y+ and system y+L transporters could be involved in the CNS to blood efflux of ADMA that we have previously observed. The half-saturation constant (Km) and maximal influx rate of the saturable component (Vmax) for [3H]-ADMA transport into the frontal cortex was 29.07{+/-}7.19 mM and 0.307{+/-}0.017 nmol.min-1.g-1, respectively, and into the CSF was 30.59{+/-}25.41 M and 2.07{+/-}0.38 nmol.min-1.g-1, respectively. This information could help explain the arginine paradox providing evidence that ADMA interacts with transporters that can remove ADMA from cells. These removal mechanisms could be stimulated by excess arginine in the plasma resulting in increased NO production. It remains to be seen if arginine supplementation could be used to increase NO production and improve hypoperfusion observed in disease states such as Alzheimers and stroke.