AMFR provides an ERAD bypass mechanism to maintain proteostasis under canonical E3 ligase deficiency
AMFR provides an ERAD bypass mechanism to maintain proteostasis under canonical E3 ligase deficiency
Nakayamada, U.; Fujii, A.; Katoh, S.; Kamada, Y.; Okiyoneda, T.
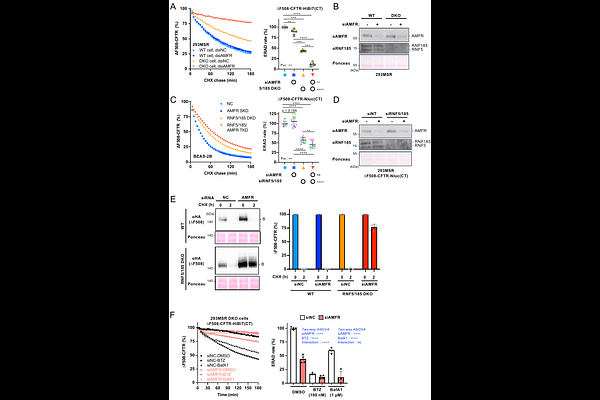
AbstractAbnormal proteins in the endoplasmic reticulum (ER) are eliminated via distinct ER-associated degradation (ERAD) pathways, each regulated by specific E3 ubiquitin ligases. While these pathways seem to work cooperatively to maintain ER proteostasis, their individual roles and potential compensatory mechanisms remain poorly defined in mammalian cells. In this study, we utilized multiple E3 ligase knockouts/knockdowns combined with a highly sensitive HiBiT-based ERAD assay to investigate pathway complementarity. We discovered that in the absence of RNF5/185 function, AMFR, a Hrd1 ortholog primarily involved in the ERAD-M branch, could partially compensate by facilitating degradation of mutant CFTR. In contrast, SYVN1, another Hrd1 ortholog, failed to show a similar effect. These findings reveal a novel bypass mechanism mediated by AMFR and demonstrate the functional flexibility of the ERAD network. Our results provide new insight into how E3 ligases maintain proteostasis under compromised conditions, advancing our understanding of ERAD pathway coordination in mammalian systems.