Interplay between SpaO variants shapes the architecture of the Salmonella type III secretion sorting platform
Interplay between SpaO variants shapes the architecture of the Salmonella type III secretion sorting platform
Soto, J. E.; Wang, T.; Galan, J. E.; Lara-Tejero, M.
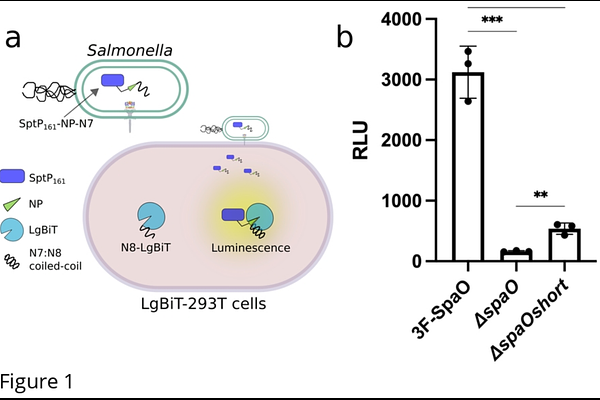
AbstractSalmonella enterica utilizes a virulence-associated type III secretion system (T3SS) to inject bacterial effectors directly into host cells. Central to this machinery is the sorting platform (SP), a cytosolic assembly whose core scaffolding protein, SpaO, is produced in two isoforms: a full-length (SpaOL) and a shorter variant (SpaOshort) comprising the C-terminal 101 residues of SpaOL. Although SpaOshort is evolutionarily conserved across type III secretion systems, its precise function has remained elusive. Here, we combined a sensitive, real-time translocation assay with site-directed photo-crosslinking to elucidate the role of SpaOshort in Salmonella SPI-1 T3SS. We found that while SpaOshort is not absolutely required for effector secretion, its absence significantly dampens T3SS-mediated protein delivery. Further biochemical and structural probing revealed that SpaOshort is a structural component of the sorting platform, arranged as a homodimer associated to SpaOL via an N-terminal \"docking motif.\" This interaction occurs while SpaOL is associated with other SP components, supporting a model in which SpaOshort is integrated into the SP pods alongside SpaOL, OrgA, and OrgB. Collectively, these findings show that SpaOshort, while not strictly essential, functions as a critical structural component of the sorting platform, providing new insights into how Salmonella and related bacteria assemble and maintain these specialized protein-injection systems.