Maternal total sleep deprivation causes oxidative stress and mitochondrial dysfunction in oocytes associated with fertility decline in mice
Maternal total sleep deprivation causes oxidative stress and mitochondrial dysfunction in oocytes associated with fertility decline in mice
Yi, Z.; Liang, Q.-x.; Zhou, Q.; Lin, Y.; Meng, Q.-r.; Li, J.; Lin, Y.; Zhang, C.; Schatten, H.; Qiao, J.; Sun, Q.-Y.
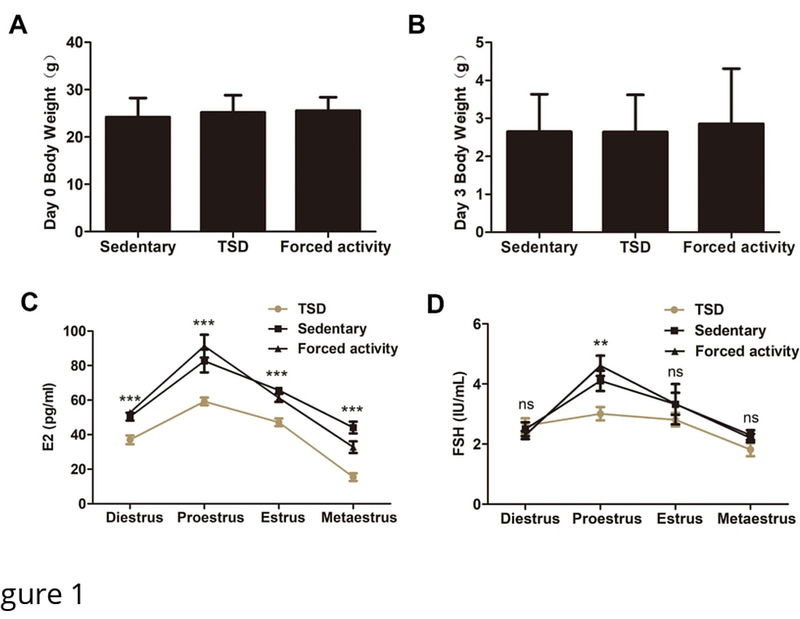
AbstractPrevious studies have shown sleep deprivation has an impact on several aspects of health and disease. Sleep deprivation suppresses melatonin production and excessive HPA activation in women, which may reduce the chances of fertility. However, little is known about how and by what relevant mechanisms it affects female fertility. In this study, female mice underwent 72 hours of total sleep deprivation (TSD) caused by rotating wheel or 2 different controls: a stationary wheel, or forced movement at night. Even though, there was no evident anomaly of natural ovulation, the TSD female mice showed impaired early embryo development. Overall levels of estrogen and FSH were lower throughout the estrus cycle (especially in proestrus). RNA Sequencing (RNA-Seq) was used to discover differentially expressed genes (DEGs) that might be involved in oocyte quality after TSD. A total of 42 genes showed significant differential expression in GV oocytes after TSD. These included genes such as Usmg5, Atp5k, Ccnd2 and Tpm3, which were enriched in gene ontology terms of mitochondrial protein complex, oxidoreductase activity, cell division, cell cycle G1/S phase transition, as well as others. The increased concentrations of reactive oxygen species (ROS) in germinal vesicle (GV) and metaphase II (MII) oocytes from TSD mice were observed, which might be induced by impaired mitochondrial function caused by TSD. The GV oocytes displayed increased mitochondrial DNA (mtDNA) copy number and a significant transient increase in inner mitochondrial membrane potential ({bigtriangleup}{psi}m) from the TSD mice probably due to compensatory effect. In contrast, MII oocytes in the TSD group showed a decrease in the mtDNA copy number and a lower delta psi m compared with the controls. Furthermore, abnormal distribution of mitochondria in the GV and MII oocytes was also observed in TSD mice, suggesting mitochondrial dysfunction. In addition, abnormal spindle and abnormal arrangement of chromosomes in MII oocytes were markedly increased in the TSD mice compared with the control mice. In conclusion, our results suggest that TSD significantly alters the oocyte transcriptome, contributing to oxidative stress and disrupted mitochondrial function, which then resulted in oocyte defects and impaired early embryo development in female mice.


