Host-derived oxidized phospholipids initiate effector-triggered immunity fostering lethality upon microbial encounter.
Host-derived oxidized phospholipids initiate effector-triggered immunity fostering lethality upon microbial encounter.
Di Gioia, M.; Poli, V.; Tan, P.; Spreafico, R.; Chou, A.; Cuenca, A.; Gordts, P.; Pandolfi, L.; Meloni, F.; Witztum, J.; Chou, J.; Springstead, J.; Zanoni, I.
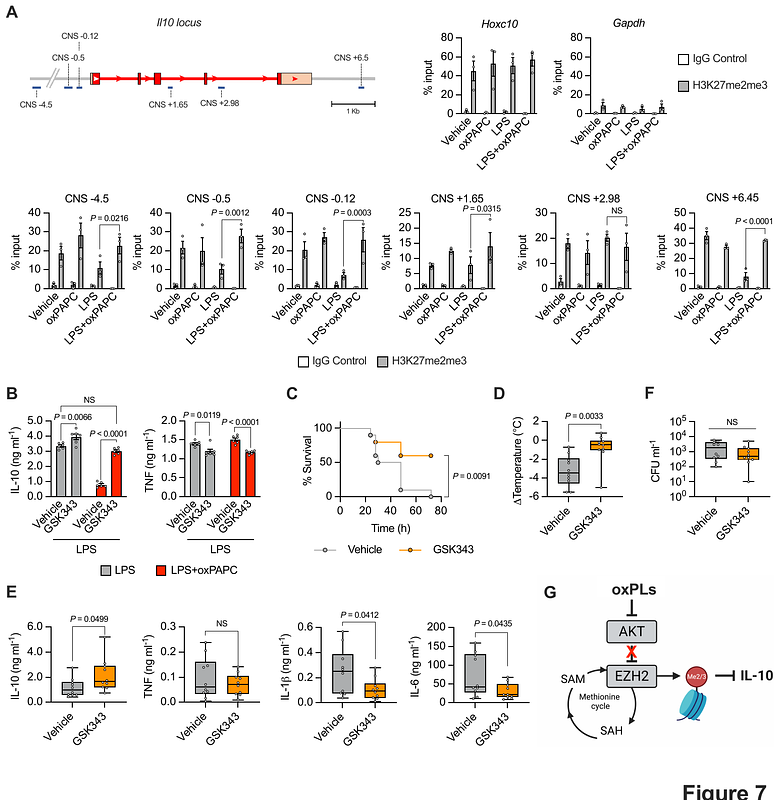
AbstractMacrophages detect invading microorganisms via pattern recognition receptors that recognize pathogen-associated molecular patterns, or via sensing the activity of virulence factors that initiates effector-triggered immunity (ETI). Tissue damage that follows pathogen encounter leads to the release of host-derived factors that participate to inflammation. How these self-derived molecules are sensed by macrophages and their impact on immunity remain poorly understood. Here we demonstrate that, in mice and humans, host-derived oxidized phospholipids (oxPLs) are formed upon microbial encounter. oxPL blockade restricts inflammation and prevents the death of the host, without affecting pathogen burden. Mechanistically, oxPLs bind and inhibit AKT, a master regulator of immunity and metabolism. AKT inhibition potentiates the methionine cycle, and epigenetically dampens Il10, a pluripotent anti-inflammatory cytokine. Overall, we found that host-derived inflammatory cues act as self virulence factors that initiate ETI and that their activity can be targeted to protect the host against excessive inflammation upon microbial encounter.