The iMab antibody selectively binds to intramolecular and intermolecular i-motif structures
The iMab antibody selectively binds to intramolecular and intermolecular i-motif structures
Ruggiero, E.; Marusic, M.; Zanin, I.; Pena Martinez, C. D.; Plavec, J. D.; Christ, D.; Richter, S.
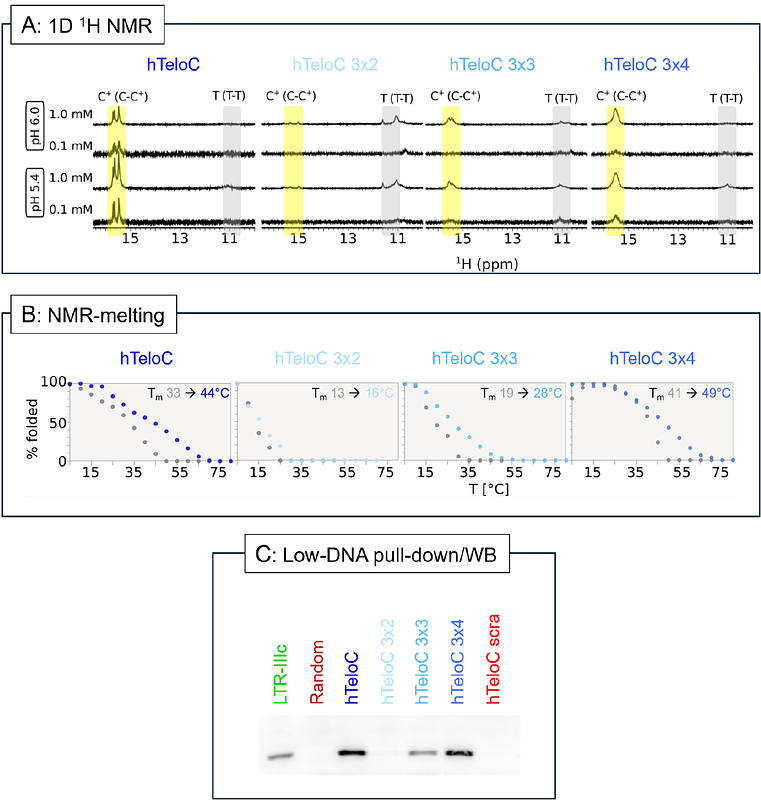
Abstracti-Motifs are quadruplex nucleic acid conformations that form in cytosine-rich regions. Because of their acidic pH dependence, iMs were thought to form only in vitro. The recent development of an iM-selective antibody, iMab, has allowed iM detection in cells, which revealed their presence at gene promoters and their cell cycle dependence. However, recently evidence emerged which seemed to suggest that iMab recognizes C-rich sequences regardless of their iM conformation. To further investigate the selectivity of iMab, we examined the binding of iMab to C-rich sequences, using a combination of pull-down and Western blot assays. Here we observe that the composition of buffers used during binding and washing steps strongly influences the selectivity of antibody binding. In addition, we demonstrate by NMR that several of the previously reported C-rich sequences, which were not expected to form iMs, actually form intermolecular iMs which are selectively recognized by iMab. Our results highlight the specificity of the iMab antibody, emphasize the importance of optimizing DNA concentrations, blocking and washing conditions, and confirm iMab selectivity not only for intramolecular iMs, but also for intermolecular iMs.


