A simplified disease resistance assay using YFP-expressing Potato Virus X in N. benthamiana reveals a cell death-independent immune function of RBA1
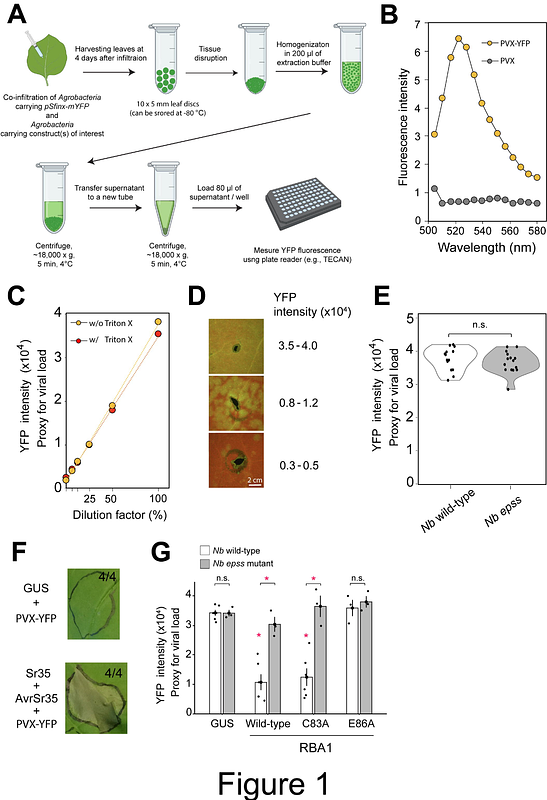
A simplified disease resistance assay using YFP-expressing Potato Virus X in N. benthamiana reveals a cell death-independent immune function of RBA1
Hasegawa, K.; Timmers, T.; Chai, J.; Maekawa, T.
AbstractR (resistance) proteins, such as intracellular NLRs (nucleotide-binding leucine-rich repeat receptors), are integral components of the plant innate immune system. Host responses following R protein activation include the generation of reactive oxygen species, sustained increases in cytosolic Ca2+, transcriptional reprogramming and, typically, rapid host cell death at sites of pathogen infection, which together ultimately lead to pathogen growth restriction. To assess the activity of R proteins, agroinfiltration-mediated transient gene expression assays have been widely used in Nicotiana species (e.g., N. benthamiana). In these transient assays, host cell death is often chosen as an indicator of R protein activity from the host responses mentioned above, in part because of the ease of experimentation. However, the extent to which host cell death is a proxy for disease resistance signaling has long been debated, as host cell death and pathogen growth restriction can be uncoupled in several cases. To assess the disease resistance activity of R proteins, bacterial growth assays have been employed in combination with transient R gene expression in N. benthamiana. Bacterial growth assays, however, require multiple experimental procedures, including agroinfiltration, pathogen infection and bacterial counts, which hinders high-throughput studies of R gene-mediated disease resistance. Here, we report a simple plate reader-based assay to assess R gene-mediated disease resistance activity against PVX (Potato virus X) that expresses YFP (PVX-YFP). Unlike bacterial pathogens, PVX proliferation in N. benthamiana is not restricted by the intrinsic activity of the EDS1 signaling pathway as previously shown by virus-induced NbEDS1 gene silencing and as we consistently show in this study using a Nbeds1 gene knockout mutant. This feature would increase the sensitivity of the assay, allowing it to capture a weak-to-moderate disease resistance activity of R proteins, as the contribution of basal immunity to PVX via the NbEDS1 pathway is negligible. Using this assay, we show that a non-cell death-inducing mutant of the R protein of RBA1 (Response to HopBA1), which lacks 2\',3\'-cAMP/cGMP synthetase activity but retains NADase activity, confers PVX resistance in an EDS1 signaling pathway-dependent manner.