Discovery of CMX990: A Potent SARS-CoV-2 3CL Protease Inhibitor Bearing a Novel Covalent Warhead
Discovery of CMX990: A Potent SARS-CoV-2 3CL Protease Inhibitor Bearing a Novel Covalent Warhead
Elshan, N. G. R. D.; Wolff, K. C.; Riva, L.; Woods, A. K.; Grabovyi, G.; Wilson, K.; Rahimi, A.; Pedroarena, J.; Ghorai, S.; Gupta, A. K.; Nazarian, A.; Weiss, F.; Liu, Y.; Mazumdar, W.; Song, L.; Okwor, N.; Malvin, J.; Bakowski, M. A.; Beutler, N.; Kirkpatrick, M. G.; Gebara-Lamb, A.; Huang, E.; Nguyen-Tran, V.; Chi, V.; Li, S.; Rogers, T. F.; McNamara, C. W.; Chen, J. J.; Joseph, S. B.; Schultz, P. G.; Chatterjee, A. K.
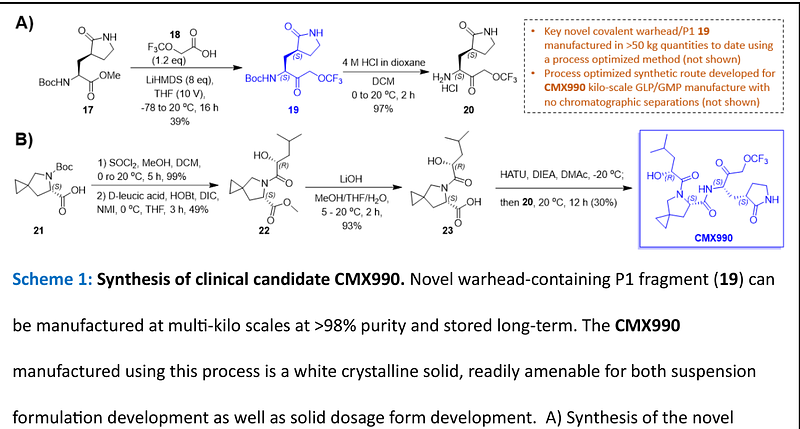
AbstractThere remains a need to develop novel SARS-CoV-2 therapeutic options that improve upon existing therapies by increased robustness of response, fewer safety liabilities, and global-ready accessibility. Functionally critical viral main protease (Mpro, 3CLpro) of SARS-CoV-2 is an attractive target due to its homology within the coronaviral family, and lack thereof towards human proteases. In this disclosure, we outline the advent of a novel SARS-CoV-2 3CLpro inhibitor, CMX990, bearing an unprecedented trifluoromethoxymethyl ketone warhead. Compared with the marketed drug nirmatrelvir (combination with ritonavir = Paxlovid), CMX990 has distinctly differentiated potency (~5x more potent in primary cells) and human in vitro clearance (>4x better microsomal clearance and >10x better hepatocyte clearance), with good in vitro-in vivo correlation. Based on its compelling preclinical profile and projected once or twice a day dosing supporting unboosted oral therapy in humans, CMX990 advanced to a Phase 1 clinical trial as an oral drug candidate for SARS-CoV-2.