Phase Separation and a Hydrodynamic Instability Localize Proteins at Growing Microtubule Ends
Phase Separation and a Hydrodynamic Instability Localize Proteins at Growing Microtubule Ends
Schaer, J.; Miesch, J.; Catapano, V.; Dumoulin, L.; Tran, J.; Velluz, M.-C.; Aumeier, C.; Kruse, K.
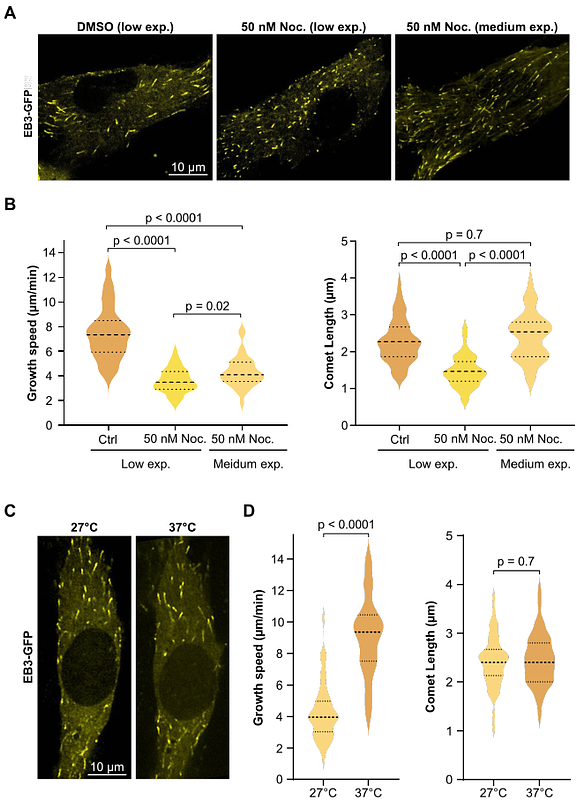
AbstractRegulating microtubule dynamics is essential for cellular function, and precise localization of regulatory proteins at microtubule ends is critical. End-binding protein EB3, a key regulator of microtubule growth, accumulates over an extended region at the growing end, forming a comet that gradually fragments into transient droplets along the microtubule shaft. Here, we combine in vitro reconstitution experiments with theoretical analysis to show that surface-mediated condensation of EB3 effectively localizes the protein at microtubule ends. Our results reveal that a Rayleigh-Plateau instability limits the condensate\'s extent, producing a finite comet length and discrete droplets along the shaft. Remarkably, the comet size is independent of the GTP-cap size. This finding is supported by experiments on cells showing that modulation of microtubule growth velocity and hence GTP-cap size do not consistently alter EB3 comet length. Furthermore, our theory shows that rapid droplet evaporation requires a transition of EB3 to a non-phase-separating state. Overall, our work challenges the view that comet length directly reflects GTP-cap size and highlights a novel mechanism for regulating microtubule dynamics.