HIV inhibits Warburg metabolism in human macrophages infected with Mycobacterium tuberculosis.
HIV inhibits Warburg metabolism in human macrophages infected with Mycobacterium tuberculosis.
Brown, K.; Walsh, A.; Yennemadi, A. S.; Murphy, D. M.; Conolly, S.; O'Sullivan, M. P.; Basdeo, S.; O'Leary, S.; Leisching, G.; Keane, J.
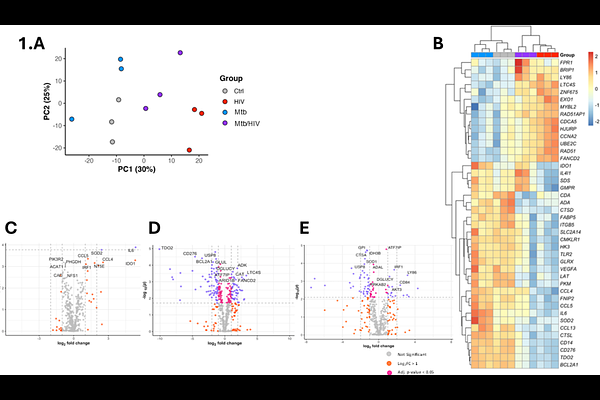
AbstractTuberculosis (TB)-associated mortality remains disproportionately high among people living with HIV (PLWH), with macrophage dysfunction representing a key mechanism of impaired host defence against Mycobacterium tuberculosis (Mtb) infection. Using the U1 chronically HIV-infected macrophage cell line model coupled with primary human monocyte-derived macrophages (MDMs) exposed to HIV-1 gp120, we systematically characterized immunometabolic perturbations during Mtb infection. Nanostring RNA analysis revealed that Mtb monoinfection upregulated glycolytic genes while suppressing oxidative phosphorylation (OXPHOS) transcripts, consistent with a Warburg-type metabolic shift. Conversely, HIV infection downregulated glycolytic enzymes and enhanced mitochondrial respiratory chain components. Coinfection studies demonstrated HIV-mediated suppression of Mtb-induced glycolytic reprogramming. Extracellular flux analysis demonstrated that gp120 exposure increased basal oxygen consumption rate while impairing spare respiratory capacity in Mtb-infected MDMs, effectively blocking the Warburg metabolic transition. Notably, gp120 concentrations equivalent to those observed in antiretroviral therapy (ART)-treated PLWH significantly disrupted metabolic plasticity and high-dose gp120 attenuated Mtb-induced TNF- secretion.