Cold-activated hepatocyte derived exosomes promote BAT thermogenesis through miR-293-5p
Cold-activated hepatocyte derived exosomes promote BAT thermogenesis through miR-293-5p
Gao, X.; Xu, J.; Xu, Z. q.; Jiang, M.; Zhu, J.; Geng, Y.; Dong, S.; Zhou, Z.; Li, Y. n.; Xu, Y. J.
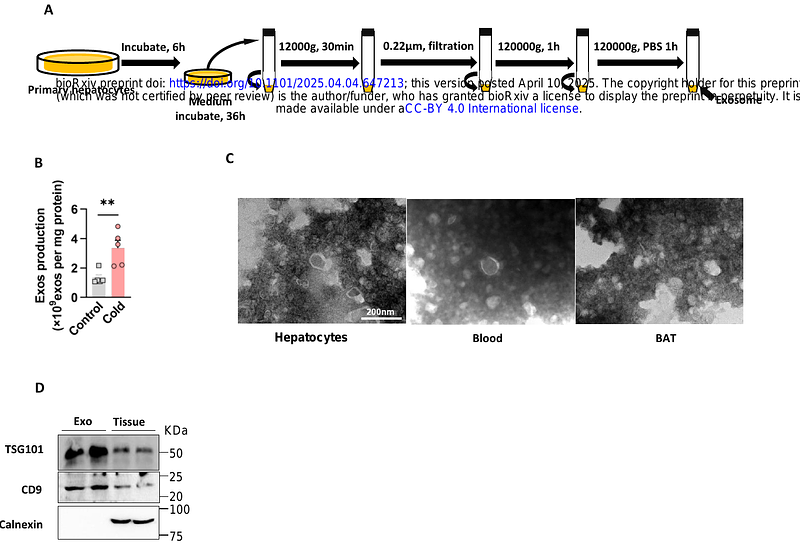
AbstractThe liver-adipose axis serves as a fundamental regulatory network governing systemic lipid homeostasis, with hepatic-derived signals orchestrating adipose tissue plasticity through multimodal mechanisms. Comprehensive elucidation of these bidirectional crosstalk mechanisms may reveal novel therapeutic strategies for metabolic disorders. Our investigation demonstrates that cold exposure induces hepatic secretion of exosomes that potentiate adipose thermogenic activation in both in vitro and in vivo models. This thermogenic potentiation is mechanistically linked to cold-induced upregulation of hepatocyte-derived exosomal miR-293-5p. Notably, pharmacological administration of miR-293-5p agomir significantly attenuates diet-induced obesity and associated metabolic dysregulation in murine models. Through mechanistic interrogation, we identified Tet1 as a direct downstream target of miR-293-5p, where ectopic Tet1 expression disrupts brown adipose tissue (BAT) thermogenic programming independently of miR-293-5p modulation. Our findings establish cold-activated hepatocyte exosomes as endocrine signaling mediators containing thermogenic microRNA cargos, with miR-293-5p emerging as a regulatory nexus coordinating mitochondrial bioenergetics and systemic energy equilibrium through liver-adipose cross-talk.