Spatially-resolved transcriptomics of human dentate gyrus across postnatal lifespan reveals heterogeneity in markers for proliferation, extracellular matrix, and neuroinflammation
Spatially-resolved transcriptomics of human dentate gyrus across postnatal lifespan reveals heterogeneity in markers for proliferation, extracellular matrix, and neuroinflammation
Ramnauth, A. D.; Tippani, M.; Divecha, H. R.; Papariello, A. R.; Miller, R. A.; Pattie, E. A.; Kleinman, J. E.; Maynard, K. R.; Collado-Torres, L.; Hyde, T. M.; Martinowich, K.; Hicks, S. C.; Page, S. C.
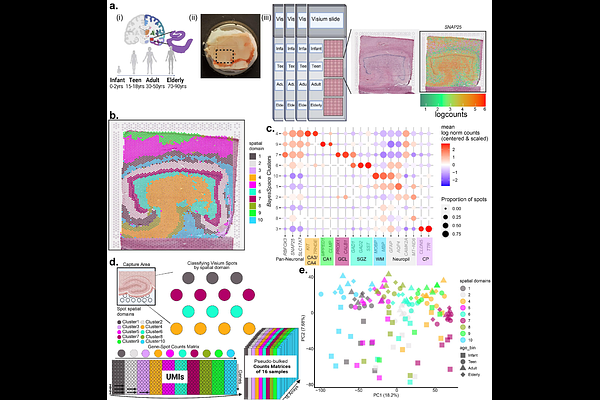
AbstractThe dentate gyrus of the anterior hippocampus is important for many human cognitive functions, including regulation of learning, memory, and mood. However, the postnatal development and aging of the dentate gyrus throughout the human lifespan has yet to be fully characterized in the same molecular and spatial detail as other species. Here, we generated a spatially-resolved molecular atlas of the dentate gyrus in postmortem human tissue using the 10x Genomics Visium platform to retain extranuclear transcripts and identify changes in molecular topography across the postnatal lifespan. We found enriched expression of extracellular matrix markers during infancy and increased expression of GABAergic cell-type markers GAD1, LAMP5, and CCK after infancy. While we identified a conserved gene signature for mouse neuroblasts in the granule cell layer (GCL), many of those genes are not specific to the GCL, and we found no evidence of signatures for other granule cell lineage stages at the GCL post-infancy. We identified a wide-spread hippocampal aging signature and an age-dependent increase in neuroinflammation associated genes. Our findings suggest major changes to the putative neurogenic niche after infancy and identify molecular foci of brain aging in glial and neuropil enriched tissue.