High-resolution respirometry reveals altered mammalian tissue ketone body oxidation in different cardiometabolic diseases
High-resolution respirometry reveals altered mammalian tissue ketone body oxidation in different cardiometabolic diseases
Zweck, E.; Piel, S.; Schmidt, J.; Scheiber, D.; Schoen, M.; Kahl, S.; Burkart, V.; Dewidar, B.; Remus, R.; Chadt, A.; Al-Hasani, H.; Aubin, H.; Boeken, U.; Lichtenberg, A.; Polzin, A.; Kelm, M.; Westenfeld, R.; Wagner, R.; Szendroedi, J.; Roden, M.; Granata, C.
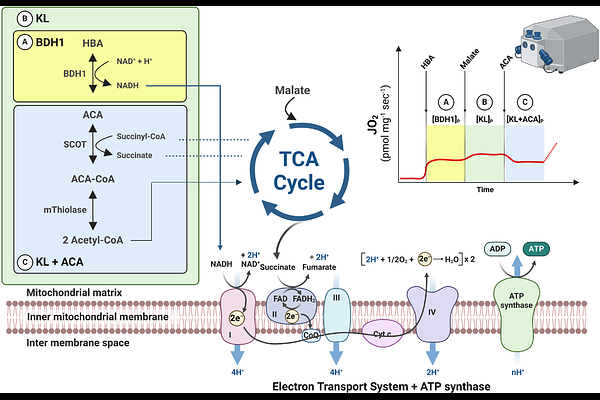
AbstractAbnormal mitochondrial oxidative phosphorylation (OXPHOS) is key to the pathogenesis of several cardiometabolic diseases. The ketone bodies (KBs), beta-hydroxybutyrate (HBA) and acetoacetate (ACA), are critical for tissue-specific energy metabolism under various pathophysiological conditions. However, robust methods quantifying their contribution as substrates for OXPHOS are lacking. Here, we first established comprehensive high-resolution respirometry protocols for assessing the differential contributions of HBA, ACA, and related ketolytic enzymes to OXPHOS in translational studies in mice and humans. We then utilized these protocols to demonstrate (i) organ-specific differences in KB-driven mitochondrial respiration in mice, (ii) lower KB-driven mitochondrial respiration in liver of humans with steatosis, skeletal muscle of humans with diabetes and in kidney of diet-induced obese mice, as well as (iii) higher mitochondrial KB utilization capacity in mouse and human failing heart. These results highlight organ-specific roles of KB metabolism in cardiometabolic diseases and shall help to identify novel targets in these pathways.