CryoET reveals actin filaments within platelet microtubules
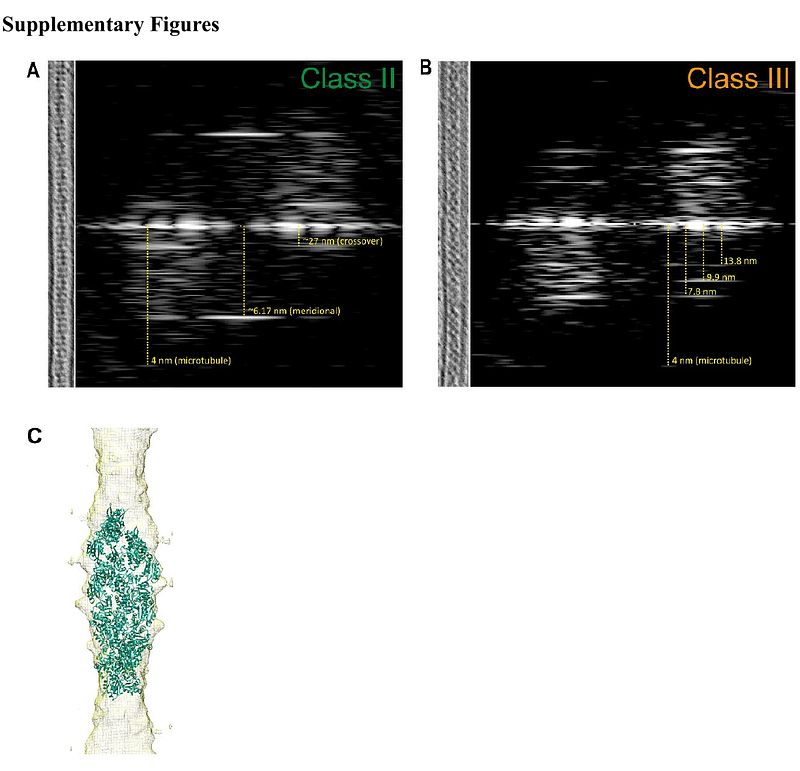
CryoET reveals actin filaments within platelet microtubules
Tsuji, C.; Bradshaw, M.; Allen, M.; Jackson, M. L.; Mantell, J.; Borucu, U.; Poole, A. W.; Verkade, P.; Hers, I.; Paul, D. M.; Dodding, M.
AbstractCrosstalk between the actin and microtubule cytoskeletons is essential for many cellular processes. Recent studies have shown that microtubules and F-actin can assemble to form a composite structure where F-actin occupies the microtubule lumen. Whether these cytoskeletal hybrids exist in physiological settings and how they are formed is unclear. Here, we show that the short-crossover Class I actin filament previously identified inside microtubules in human HAP1 cells is cofilin-bound F-actin. Lumenal F-actin can be reconstituted in vitro, but cofilin is not essential. Moreover, actin filaments with both cofilin-bound and canonical morphologies reside within human platelet microtubules under physiological conditions. We propose that stress placed upon the microtubule network during motor-driven microtubule looping and sliding may facilitate the incorporation of actin into microtubules.