Inhibition of CELA1 Improves Septation in the Mouse Hyperoxia Model of Impaired Alveolar Development
Inhibition of CELA1 Improves Septation in the Mouse Hyperoxia Model of Impaired Alveolar Development
Smith, N. J.; Joshi, R.; Desmukh, H.; Gray, J.; Edwards, A. D.; Shahreki, E.; Varisco, B. M.
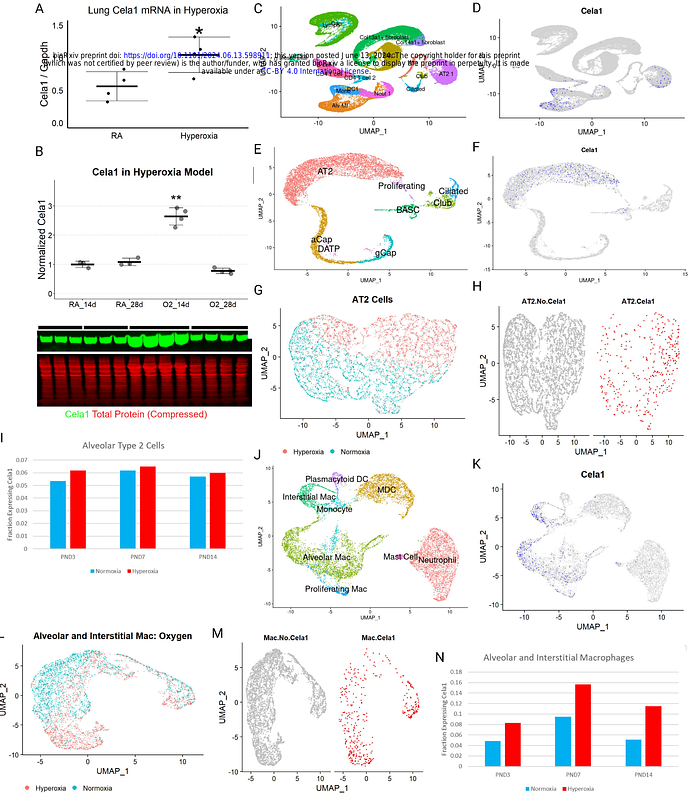
AbstractA key feature of bronchopulmonary dysplasia (BPD) is impaired alveolar septation. In later live, BPD survivors are more susceptible to childhood respiratory problems and have reduced respiratory function as adults. Chymotrypsin-like elastase 1 (CELA1) is a serine protease expressed in AT2 cells that mediates emphysema progression in adult mouse models. CELA1 binds and cleaves tropoelastin in response to strain. Its expression is developmentally regulated. Using the mouse hyperoxia model of impaired alveolar development we hypothesized a role for CELA1 in impaired alveolar development (IAD). In C57BL6 mouse pup lungs exposed to 80% oxygen for 14 days Cela1 mRNA increased 1.9-fold (p<0.05) and protein 2.6-fold (p<0.01). Protein levels normalized after 14 days in room air. Analysis of an existing single cell mRNA-seq dataset showed Cela1 mRNA in AT2 cells, alveolar macrophages and interstitial macrophages. The fraction of cells with Cela1 mRNA increased with hyperoxia. By flow cytometry the only Cela1-specific difference in immune cell populations was a 2-fold increase in lung eosinophils in room air (p<0.05). After 14 days of exposure to 80% oxygen Cela1-/- mice had better alveolarization with an average mean linear intercept of 80 um compared to 111um (p<0.001). Treatment of hyperoxia-exposed pups with subcutaneous anti-Cela1 KF4 antibody offered similar protection compared to IgG (59 um vs. 67 um, p<0.001). Human BPD specimens demonstrated CELA1 in AT2 cells and myeloid cells. These data indicate that hyperoxia-induced increases in CELA1 are partially responsible for IAD and suggest a potential role in premature neonates exposed to high FiO2.


