Light and sex modify Snord116 genotype effects on metabolism, behavior, and imprinted gene networks following circadian entrainment
Light and sex modify Snord116 genotype effects on metabolism, behavior, and imprinted gene networks following circadian entrainment
Mendiola, A. J. P.; Rutkowsky, J. M.; Neier, K. E.; Hakam, S.; Sharifi, O.; Donnelly, C.; Torres, C.; Hernandez, C.; Yasui, D. H.; Ramsey, J. J.; LaSalle, J. M.
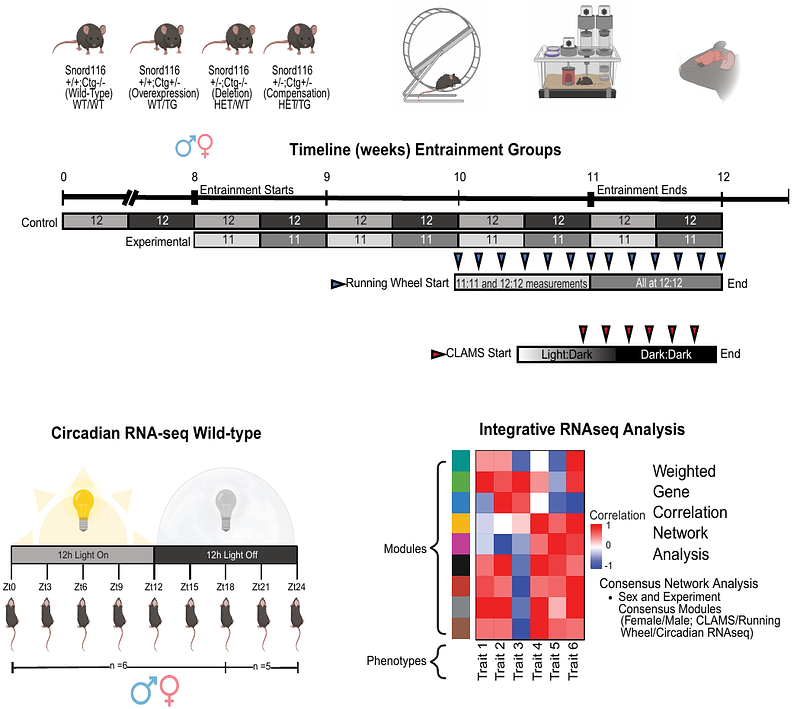
AbstractMammals utilize imprinted and X-linked epigenetic mechanisms in development, metabolism, and behavior. Imprinted genes, including Prader-Willi syndrome Snord116 noncoding RNAs, are implicated in the regulation of sleep and circadian rhythms through poorly understood mechanisms. Utilizing mouse models of Snord116 deficiency and overexpression, we performed an integrated, sex-stratified analysis of free running behaviors, indirect calorimetry, and cortical transcriptomes following entrainment to a 22 hour light:dark T-cycle. We observed significant interactions of sex, entrainment, and Snord116 genotype in period length at baseline and after-effects of post-entrainment. Snord116 deletion effect on respiratory exchange ratio was light sensitive, with sex and entrainment effects dominant under total darkness. Snord116 genotype impacted both rhythmic and non-rhythmic cortical gene networks that integrated sex, light, and entrainment effects with genotype-phenotype correlations. A co-expressed gene network enriched for imprinted, Snord116-target, and Xist-proximal long noncoding RNAs was identified as a light-sensitive regulatory hub of sexual dimorphic responses to a dynamic environment.