3D osteocyte networks under Pulsatile Unidirectional Fluid Flow Stimuli (PUFFS)
3D osteocyte networks under Pulsatile Unidirectional Fluid Flow Stimuli (PUFFS)
Merife, A.-B.; Poudel, A.; Polshchikova, A.; Geffert, Z. J.; Horton, J.; Akash, M. M. H.; Pandey, A.; Basu, S.; Fougnier, D.; Soman, P.
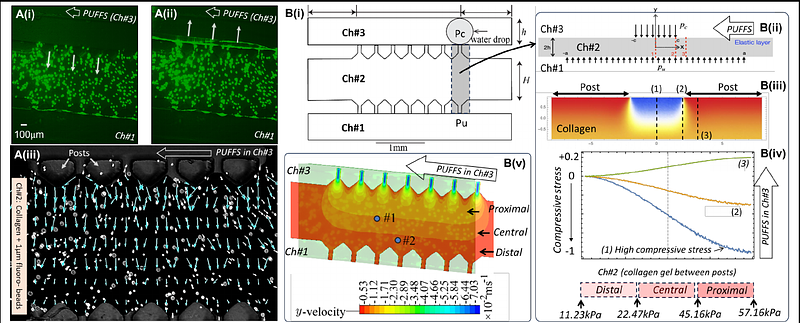
AbstractAlthough osteocytes are known to play a key role in skeletal mechano-adaptation, few in vitro models have investigated how pulsatile mechanical stimuli influence the properties of 3D osteocyte networks. Here we design and develop a microfluidic based in vitro model to study 3D osteocyte networks cultured under Pulsatile Unidirectional Fluid Flow Stimuli (PUFFS). Digital light projection stereolithography was used to design and fabricate a three-chambered PDMS microfluidic chip. Model osteocytes (murine MLO-Y4) were encapsulated in collagen matrix within the chip to form self-assembled three-dimensional (3D) cell networks. Daily stimulus in the form of PUFFS was then applied for upto 21 days. A combination of experiments, computational simulation and analytical modeling was used to characterize the mechanical environment experienced by embedded cells during PUFFS. Viability, morphology, cell-connectivity, expression of key proteins, and gene expression, and real-time calcium signaling within 3D osteocyte networks were characterized at select time-points and compared to static conditions. Results show that PUFFS stimulation at 0.33 and 1.66 Hz can initiate mechanotransduction via calcium signals that are propagated across the network of collagen encapsulated osteocytes via Cx43 junctions. Furthermore, osteocytes cultured in these devices maintain expression of several key osteocyte genes for up to 21d. Taken together, this model can potentially serve as a testbed to study how 3D osteocyte networks respond to dynamic mechanical stimulation relevant to skeletal tissues.