Inhibition of NLRP3 by a CNS-penetrating indazole scaffold
Inhibition of NLRP3 by a CNS-penetrating indazole scaffold
Torp, J.; Ferber, D.; Buthmann, H.; Hagelueken, G.; Deshpande, A.; Hartman, G.; Hughes, R. E.; Salazar, T.; Tronnes, S.; Duisembekova, A.; Marleaux, M.; Hochheiser, I. V.; Coll, R. C.; Montgomery, R.; Fortney, K.; Py, B. F.; Wilhelmsen, K.; Geyer, M.
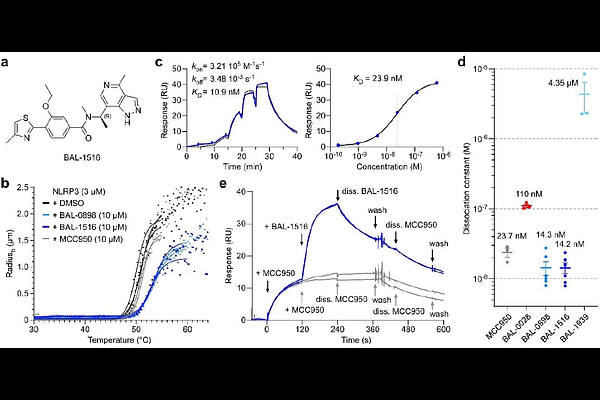
AbstractLow-grade inflammation is a hallmark of ageing and a key cause of age-related impairments and diseases. The NOD-like receptor NLRP3 senses a variety of danger signals and environmental insults, resulting in pro-inflammatory response, inflammasome formation and pyroptosis. Its aberrant activation has been linked to many acute and chronic diseases ranging from atherosclerosis to Alzheimers disease and cancer, making NLRP3 an attractive therapeutic target. Here we report the discovery, characterization, and structure of an indazole-based NLRP3 antagonist, BAL-1516, which potently inhibits inflammasome formation in monocytes and microglia. The cryo-electron microscopy structure of BAL-1516 bound to NLRP3 reveals a previously undescribed compound binding site at a surface groove of the nucleotide-binding domain with contacts to the FISNA and WHD subdomains. The characteristic feature of BAL compound binding is the formation of three hydrogen bonds to the peripheral beta-strand of the triple-ATPase; two from the indazoles nitrogen atoms and a third from the compounds linker region. Additional phenyl and thiazole moieties render the compound hydrophobic, allowing excellent blood-brain barrier penetration. The compound binding site is highly specific for NOD-like receptors, and the optimized compound BAL-1516 is able to directly bind mouse NLRP3 despite two conservative residue changes in the binding interface. The BAL compounds represent a first-in-class family of NLRP3 inhibitors, providing a broad design space, including covalent and degradative properties, for the development of NLRP3-directed therapeutics.