High-integrity nanoemulsions formulation of resiquimod (R848) enhances stability and delivery for triple negative breast cancer immunotherapy
High-integrity nanoemulsions formulation of resiquimod (R848) enhances stability and delivery for triple negative breast cancer immunotherapy
Salem, A.; Attia, S.; El-Ghlban, S.; Montaser, A. S.; Abdelhameed, M. F.; Helmy, M. W.; Attia, M. F.
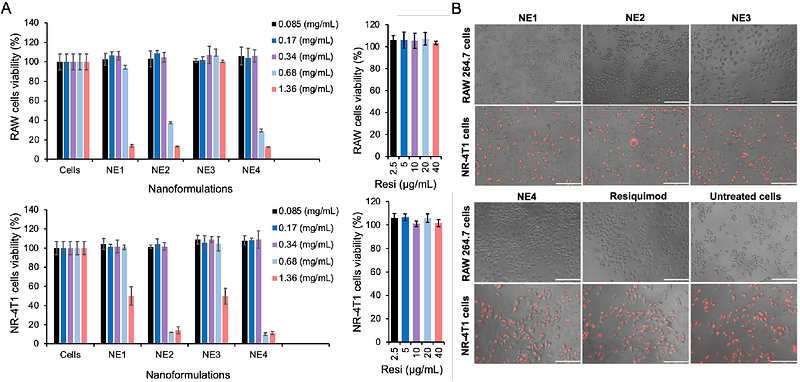
AbstractTriple-negative breast cancer (TNBC) poses significant clinical challenges due to its high heterogeneity, with multiple subtypes exhibiting distinct molecular characteristics and treatment responses. The development of resistance to chemotherapy and targeted therapies remains a major obstacle, and identifying reliable biomarkers to predict therapeutic response continues to be challenging. This study aims to enhance the delivery of the immunostimulant Toll-like receptor 7/8 (TLR7/8) agonist resiquimod (R848) using a safe and highly integrated nanoemulsions (NEs) formulation, providing effective and reliable immunotherapy. A series of NEs were prepared and optimized with and without the reactive lipophilic compound ricinoleic acid. Neutral and negatively charged NE formulations encapsulating R848 were compared. The physicochemical properties and in vitro delivery of resiquimod into RAW 264.7 macrophages and 4T1 TNBC cell line models were studied. Both R848-loaded NE formulations exhibited prolonged shelf-life stability with minimal protein binding. Incorporating small portions of ricinoleic acid into the formulation (negatively charged NEs) slowed drug release and improved physical properties and overall delivery compared to ricinoleic-free formulations, likely due to its interaction with the drug. Cytotoxicity and cellular uptake studies were conducted on both NE models, showing localization in the macrophage cell membrane and 4T1 cell cytoplasm. Molecular profiling in 4T1 cells revealed R848-NEs modulated key biomarkers (TLR4/7, Cyclin D1, NF-{kappa}B) while potently inducing autophagy (evidenced by LC3II/p62/Beclin-1 alterations) and PD-L1 upregulation. These dual effects, autophagy-mediated tumor suppression and immune checkpoint modulation, suggest therapeutic synergy between R848-NEs and anti-PD-L1 antibodies, presenting a promising combinatorial strategy for TNBC treatment.