Cellular zinc status alters chromatin accessibility and binding of transcription factor TP53 to genomic sites
Cellular zinc status alters chromatin accessibility and binding of transcription factor TP53 to genomic sites
Damon, L. J.; Ocampo, D.; Sanford, L.; Jones, T.; Allen, M. A.; Dowell, R. D.; Palmer, A. E.
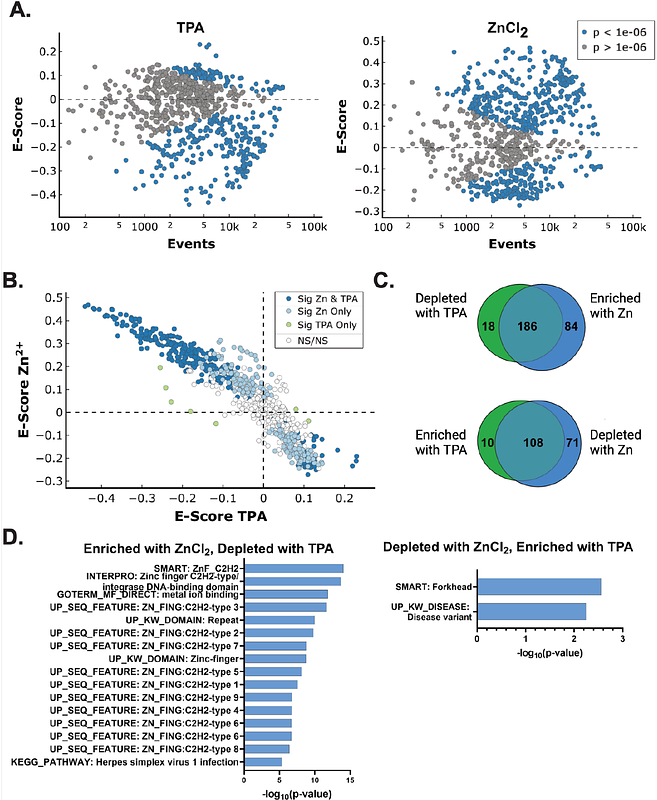
AbstractZinc (Zn2+) is an essential metal required by approximately 2500 proteins. Nearly half of these proteins act on DNA, including > 850 human transcription factors, polymerases, DNA damage response factors, and proteins involved in chromatin architecture. How these proteins acquire their essential Zn2+ cofactor and whether they are sensitive to changes in the labile Zn2+ pool in cells remain open questions. Here, we examine how changes in the labile Zn2+ pool affect chromatin accessibility and transcription factor binding to DNA. We observed both increases and decreases in accessibility in different chromatin regions via ATAC-seq upon treating MCF10A cells with elevated Zn2+ or the Zn2+-specific chelator tris(2-pyridylmethyl)amine (TPA). Transcription factor enrichment analysis was used to correlate changes in chromatin accessibility with transcription factor motifs, revealing 477 transcription factor motifs that were differentially enriched upon Zn2+ perturbation. 186 of these transcription factor motifs were enriched in Zn2+ and depleted in TPA, and the majority correspond to Zn2+ finger transcription factors. We selected TP53 as a candidate to examine how changes in motif enrichment correlate with changes in transcription factor occupancy by ChIP-qPCR. Using publicly available ChIP-seq and nascent transcription datasets, we narrowed the 50,000+ ATAC-seq peaks to 2164 TP53 targets and subsequently selected 6 high-probability TP53 binding sites for testing. ChIP-qPCR revealed that for 5 of the 6 targets, TP53 binding correlates with the local accessibility determined by ATAC-seq. These results demonstrate that changes in labile zinc directly alter chromatin accessibility and transcription factor binding to DNA.