The Golgi Rim is a Precise Tetraplex of Golgin Proteins that Can Self-Assemble into Filamentous Bands
The Golgi Rim is a Precise Tetraplex of Golgin Proteins that Can Self-Assemble into Filamentous Bands
Su, M.; Radhakrishnan, A.; Yan, Y.; Tian, Y.; Zheng, H.; M'Saad, O.; Graham, M.; Coleman, J.; Goder, J. N. D.; Liu, X.; Zhang, Y.; Bewersdorf, J.; Rothman, J. E.
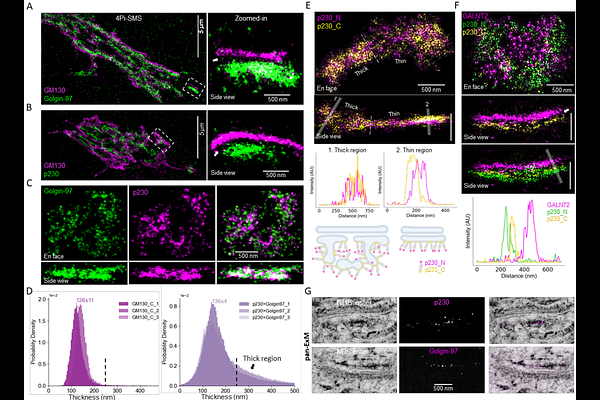
AbstractGolgin proteins have long been suspected to be organizers of the Golgi stack. Using three-dimensional super-resolution microscopy, we comprehensively localize the human golgin family at the rim of the Golgi apparatus at 10-20 nm resolution in situ. Unexpectedly, we find that the golgins are precisely organized into a tetraplex with four discrete layers, each containing a specific set of rim golgins. We observe no golgins inside the stack between its membrane-bound cisternae. Biochemically characterizing most of the golgins as isolated proteins, we find that they form anti-parallel dimers and further self-assemble into bands of multi-micron-long filaments. Based on our findings, we propose an \'outside-in\' physical model, the Golgin Organizer Hypothesis, in which the Golgi stack of cisternae and its overall ribbon morphology directly result from bending circumferential bands of rim golgin filaments onto a membrane surface, explaining stack formation without the need for special stacking proteins.