The chemical chaperone 4-phenylbutyric acid rescues molecular cell defects of COL3A1 mutations that cause vascular Ehlers Danlos Syndrome
The chemical chaperone 4-phenylbutyric acid rescues molecular cell defects of COL3A1 mutations that cause vascular Ehlers Danlos Syndrome
Omar, R.; Lee, M.; Gonzalez-Trueba, L.; Lianos, S.; Hazarika, S.; Ammar, M. A.; Cassels, J.; Michie, A. M.; Bulleid, N. J.; Malfait, F.; Van Agtmael, T.
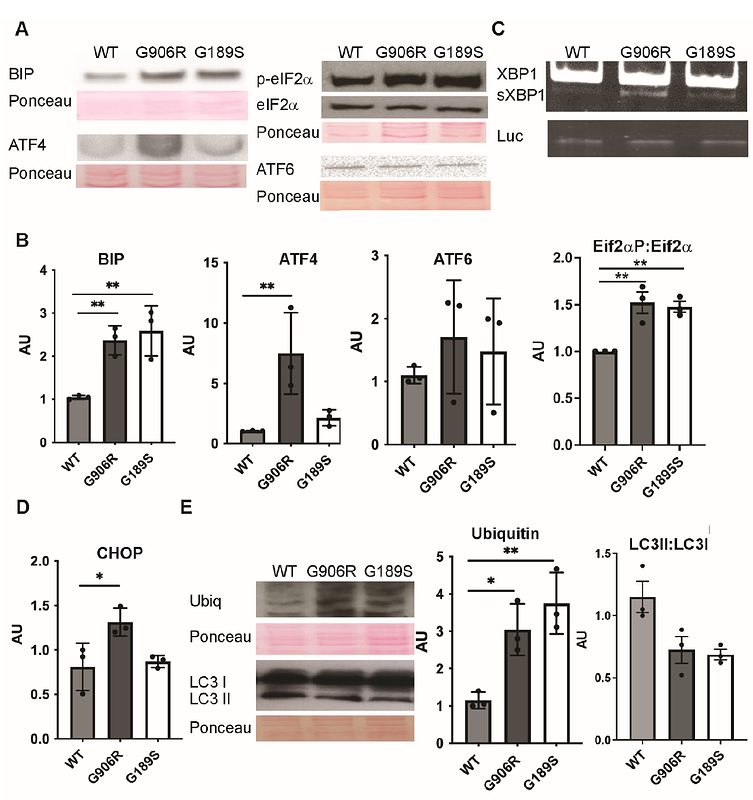
AbstractPurpose: Vascular Ehlers Danlos Syndrome (vEDS) is a connective tissue disorder caused by COL3A1 mutations for which there are no treatments due to a limited understanding of underlying mechanisms. We aimed to address this critical knowledge gap, focusing on collagen folding, to establish if targeting protein folding represents a potential therapeutic approach. Methods: We performed a mechanistic analysis of two novel COL3A1 glycine mutations, G189S and G906R, using primary patient fibroblast cultures, and performed pre-clinical proof-of-concept treatments using FDA-approved chemical chaperones targeting protein folding and/or degradation. Results: COL3A1 mutations caused secretion of misfolded collagen III and intracellular collagen retention, leading to matrix defects and endoplasmic reticulum (ER) stress, with increased severity for the more C-terminal mutation. Promoting ER protein folding capacity through the chemical chaperone 4-phenylbutyric acid rescued the ER stress, thermostability of secreted collagen, matrix defects and apoptosis. Optimising treatment duration and dosage helped overcome allele-dependent treatment efficacy. In contrast, protein degradation alone or combined with targeting protein folding did not increase efficacy. Conclusion: ER stress is a molecular mechanism in vEDS that can be influenced by the position of COL3A1 mutation, and promoting protein folding is a putative mechanism-based therapeutic approach that can rescue intra- and extracellular defects.


