Development of capsaicin derivatives as prohibitin ligands to modulate the Aurora kinase A/PHB2 interaction in cancer cells
Development of capsaicin derivatives as prohibitin ligands to modulate the Aurora kinase A/PHB2 interaction in cancer cells
Djehal, A.; Caron, C.; Pizza, V.; Farin, K.; Bertolin, G.; Desaubry, L.
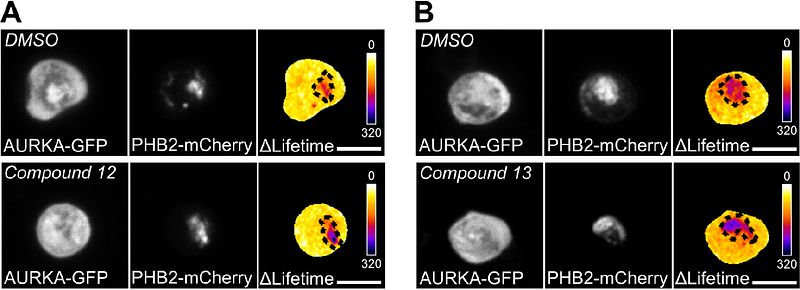
AbstractAurora kinase A/AURKA is a serine/threonine kinase overexpressed in a variety of solid and hematological malignancies. In the last decades, clinical trials aiming to counteract the overexpression of AURKA turned out to be largely unsuccessful. Meanwhile, recent discoveries pointed at new functions of AURKA at the subcellular level, including at mitochondria. At this location, AURKA induces organelle clearance by mitophagy by acting in complex with the mitophagy mediator LC3, and its inner mitochondrial membrane receptor PHB2. The natural polyphenol xanthohumol was shown to act as a PHB2 ligand, altering the interaction between AURKA and PHB2 and restoring mitochondrial functions in cancer cells. However, its chemical nature prevents its broader use as an anti-cancer agent. Using Forster\'s Resonance Energy Trasfer/Fluorescence Lifetime Imaging Microscopy (FRET/FLIM) in live breast cancer cells, we here explore the effects of alternative PHB ligands in altering the proximity between AURKA and PHB2. Among the already-available compounds, we found that the pungent natural product capsaicin partially alters the AURKA/PHB2 protein-protein proximity. We then synthesized 16 novel capsaicin analogues to enhance the effects of capsaicin. We found that replacing the long hydrophobic acyl moiety by a butyryl one increases the AURKA/PHB2 interaction. Among the capsaicin derivatives carrying this modification, we uncover that compound 13 enhances the AURKA/PHB2 proximity with a low experimental variability. Together, our data indicate that compound 13 is a promising PHB ligand acting on the AURKA/PHB2 interaction, and it may provide the basis for the development of new anticancer drugs targeting the mitochondrial functions of AURKA.


