Scalable intracellular delivery via microfluidic vortex shedding enhances the function of chimeric antigen receptor T-cells
Scalable intracellular delivery via microfluidic vortex shedding enhances the function of chimeric antigen receptor T-cells
Sytsma, B. J.; Allain, V.; Bourke, S.; Faizee, F.; Fathi, M.; Berdeaux, R.; Ferreira, L. M. R.; Brewer, J.; Li, L.; Pan, F. L.; Rothrock, A. G.; Nyberg, W. A.; Li, Z.; Wilson, L. H.; Eyquem, J.; Pawell, R. S.
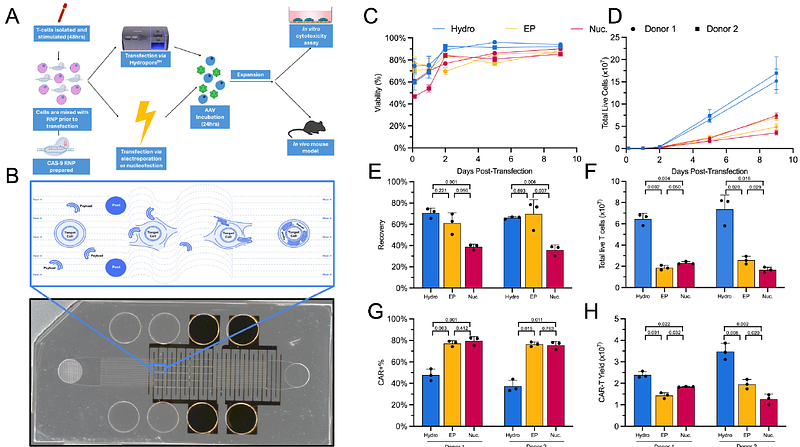
AbstractAdoptive chimeric antigen receptor T-cell (CAR-T) therapy is transformative and approved for hematologic malignancies, as well being developed for treatment of solid tumors, autoimmune disorders, heart disease and aging. Despite unprecedented clinical outcomes, CAR-T and other engineered cell therapies face a variety of manufacturing and safety challenges. Traditional methods, like lentivirus transduction and electroporation, result in random integration or cause significant cellular damage, which can limit the safety and efficacy of engineered cell therapies, such as CAR-T. We present hydroporation as a gentle and effective alternative for intracellular delivery. Hydroporation resulted in 1.7 to 2x higher CAR-T yields compared to electroporation with superior cell viability and recovery. Hydroporated cells exhibited rapid proliferation, robust target cell lysis and increased pro-inflammatory and regulatory cytokine secretion in addition to improved CAR-T yield by day 5 post-transfection. We demonstrated scaled-up hydroporation can process 5 x 108 cells in less than 10 seconds, showcasing the platform as a viable solution for high-yield, precise CAR-T cell manufacturing with the potential for improved therapeutic outcomes.


