Characterization of a COQ8A-ataxia mouse model with E548K single-site mutation: distinct and comparable findings relative to a loss-of-function mutation
Characterization of a COQ8A-ataxia mouse model with E548K single-site mutation: distinct and comparable findings relative to a loss-of-function mutation
Goncalves, D.; Broyer, N.; Carret Pierrat, M.; Sarzi Campillo, E.; Reutenauer, L.; Puccio, H.
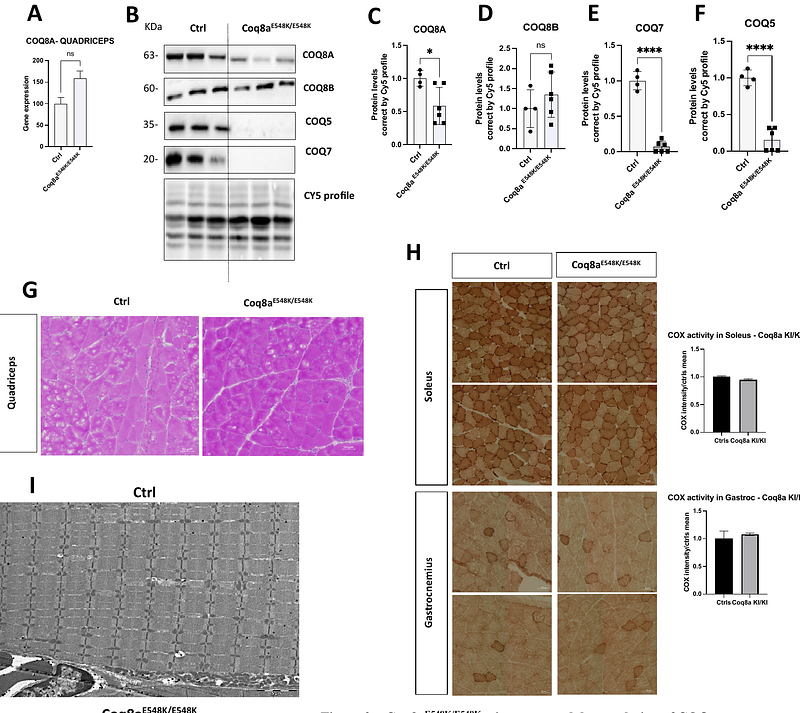
AbstractCOQ8A-ataxia, also known as autosomal recessive cerebellar ataxia type 2 (ARCA2), is a rare mitochondrial disorder caused by biallelic mutations in COQ8A, a gene encoding for a mitochondrial protein critical for coenzyme Q (CoQ) biosynthesis. Although there is no clear genotype-phenotype correlation in patients, loss-of function variants generally produce a cerebellar-restricted phenotype, while missense mutations are more frequently associated with multisystemic symptoms. The COQ8AE551K variant has been reported at the homozygous state in individuals with early-onset disease and widespread systemic involvement. This study aimed to characterize the new Coq8aE548K knocking mouse model, equivalent to the human E551K variant, and compare its phenotype to the complete Coq8a-/- knockout mouse. Based on human data and preliminary data in the zebrafish, we hypothesized that the Coq8aE548K knocking would present a more pronounced phenotype than the constitutive knockout. Contrary to our initial hypothesis, mice homozygous for the Coq8aE548K allele exhibited no significant motor or cognitive impairments, nor muscle phenotype. Biochemically, the knocking Coq8aE548K mutation led to variable instability of the COQ8AE548K protein and reduced expression of proteins in the CoQ biosynthesis pathway, such as COQ5 and COQ7 in both cerebellum and muscle, similarly to the constitutive knockout. Despite this, mitochondrial function and tissue architecture remained intact, suggesting preserved cellular resilience. These findings highlight the complexity of genotype-phenotype correlations in COQ8A-related ataxia and provides a tool for investigating sub-threshold mitochondrial dysfunction.