Salmonella Typhimurium employ spermidine to exert protection against ROS-mediated cytotoxicity and rewires host polyamine metabolism to ameliorate its survival in macrophages
Salmonella Typhimurium employ spermidine to exert protection against ROS-mediated cytotoxicity and rewires host polyamine metabolism to ameliorate its survival in macrophages
Nair, A. V.; Singh, A.; Rajmani, R. S.; Chakravortty, D.
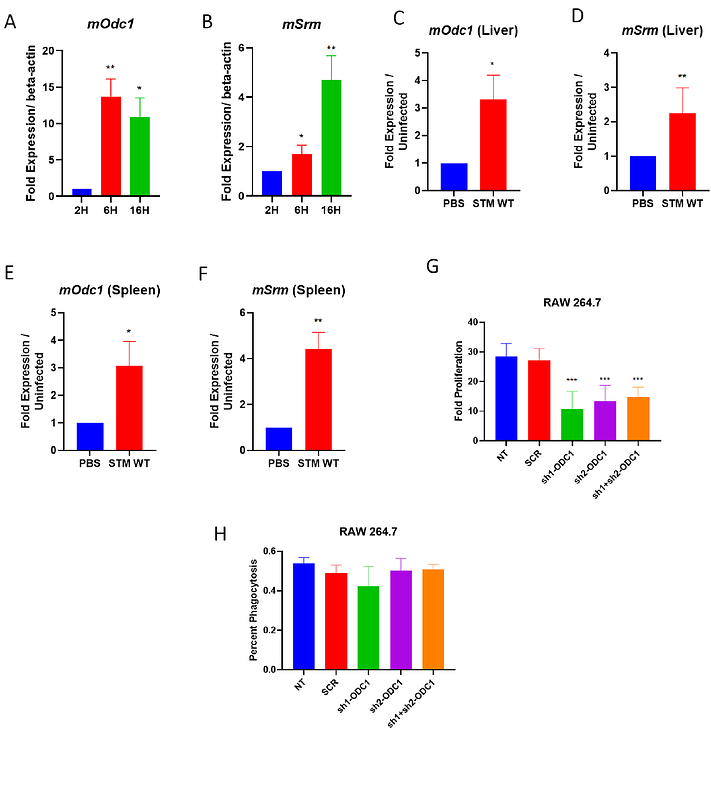
AbstractSalmonella infection involves a cascade of attacks and defence measures. After breaching the intestinal epithelial barrier, Salmonella is phagocytosed by the macrophages, inside which, the bacteria face multiple stresses and, consequently, employ appropriate countermeasures. We show that, in Salmonella, the polyamine spermidine activates a stress response mechanism by regulating critical antioxidant genes. Salmonella Typhimurium mutants for spermidine transport and synthesis cannot mount an antioxidative response, resulting in high intracellular ROS levels. These mutants are also compromised in their ability to be phagocytosed by macrophages. Furthermore, it regulates a novel enzyme in Salmonella, Glutathionyl-spermidine synthetase (GspSA), which is known to prevent the oxidation of proteins in E.coli. Moreover, the spermidine mutants and the GspSA mutant show significantly reduced survival in the presence of hydrogen peroxide in vitro, and lesser organ burden in the mouse model of Salmonella infection. Conversely, in macrophages isolated from gp91phox-/- mice, we observed a rescue in the attenuated fold proliferation previously observed upon infection. Interestingly, Salmonella upregulates polyamine biosynthesis in the host, which also solves the mystery of the attenuated proliferation observed in spermidine transport mutants. Thus, inhibition of this pathway in the host abrogates the proliferation of Salmonella Typhimurium in macrophages. From a therapeutic perspective, inhibiting host polyamine biosynthesis using an FDA-approved chemopreventive drug, D,L--difluoromethylornithine (DFMO), reduces Salmonella colonization and tissue damage in the mouse model of infection, while enhancing the survival of infected mice. Therefore, our work provides a mechanistic insight into the critical role of spermidine in stress resistance of Salmonella. It also reveals a strategy of the bacteria in modulating host metabolism to promote their intracellular survival and shows the potential of DFMO to curb Salmonella infection.