Backtranslation of human RNA biosignatures of tuberculosis disease risk into the preclinical pipeline is condition dependent
Backtranslation of human RNA biosignatures of tuberculosis disease risk into the preclinical pipeline is condition dependent
Painter, H.; Larsen, S. E.; Williams, B. D.; Abdelaal, H. F. M.; Baldwin, S. L.; Fletcher, H. A.; Fiore-Gartland, A.; Coler, R.
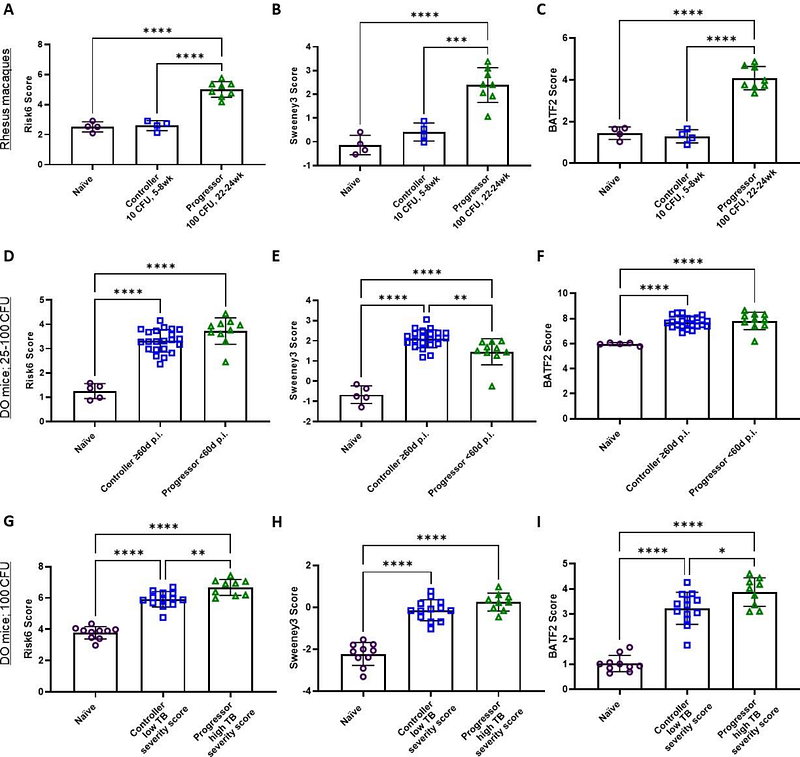
AbstractIt is not clear whether human progression to active tuberculosis disease (TB) risk signatures are viable endpoint criteria for evaluations of treatments in clinical or preclinical development. TB is the deadliest infectious disease globally and more efficacious vaccines are needed to reduce this mortality. However, the immune correlates of protection for either preventing infection with Mycobacterium tuberculosis or preventing TB disease have yet to be completely defined, making the advancement of candidate vaccines through the pipeline slow, costly, and fraught with risk. Human-derived correlate of risk (COR) gene signatures, which identify an individual\'s risk to progressing to active TB disease, provide an opportunity for evaluating new therapies for TB with clear and defined endpoints. Though prospective clinical trials with longitudinal sampling are prohibitively expensive, characterization of COR gene signatures is practical with preclinical models. Using a 3Rs (Replacement, Reduction and Refinement) approach we reanalyzed heterogeneous publicly available transcriptional datasets to determine whether a specific set of COR signatures are viable endpoints in the preclinical pipeline. We selected RISK6, Sweeney3 and BATF2 human-derived blood-based RNA biosignatures because they require relatively few genes to assign a score and have been carefully evaluated across several clinical cohorts. Excitingly, these data provide proof-of-concept that human COR signatures seem to have high fidelity across several tissue types in the preclinical TB model pipeline and show best performance when the model most closely reflected human infection or disease conditions. Human-derived COR signatures offer an opportunity for high-throughput preclinical endpoint criteria of vaccine and drug therapy evaluations.


