Differential effects of the D1/S264V mutation in Photosystem II with either PsbA1 or PsbA3 on QB, non-heme Iron, and the associated hydrogen-bond network
Differential effects of the D1/S264V mutation in Photosystem II with either PsbA1 or PsbA3 on QB, non-heme Iron, and the associated hydrogen-bond network
Tada, K.; Yamagata, K.; Koyama, K.; Selles, J.; Boussac, A.; Sugiura, M.
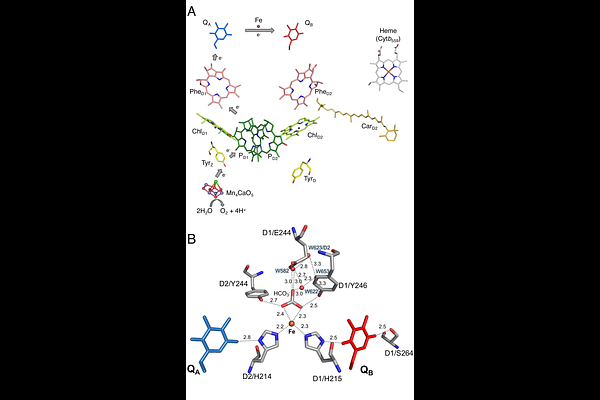
AbstractThe role of the D1/S264 residue and the role of its environment in the proton-coupled electron transfer reaction on the acceptor side of Photosystem II were investigated. To this end, D1/S264V mutants were constructed in the thermophilic cyanobacterium Thermosynechococcus elongatus, with D1 being either PsbA1 or PsbA3. The PSII mutants were investigated using EPR spectroscopy, thermoluminescence, (time-resolved) absorption changes measurements, and oximetry. While the mutation had minor effects in PsbA1-PSII, the S264V mutation in PsbA3-PSII had significant consequences: i) thermoluminescence data show inefficient electron transfer from QA- to QB; ii) re-oxidation of QA- was slowed, by at least a factor of 10; iii) the herbicides inhibit weakly O2 evolution; iv) no Fe2+QB- EPR signal was detected in dark-adapted PSII; instead, v) a large Fe3+ signal was present with vi) modified EPR properties; vii) no QA-Fe2+QB- biradical signal was observed after illumination at 198 K following a flash illumination, confirming the inefficient formation of QB-; viii) either no proton uptake coupled to non-heme iron reduction occurred or with a very slow rate compared to PsbA3-PSII; ix) changes were noted in the electrochromic response associated with QA- formation; and x) increased production of singlet oxygen, both with and without herbicides. The S264V mutation in PsbA3-PSII leads to a significant decrease in the energy gap between the QA-QB and QAQB- states. The effects listed above are discussed regarding the differences between PsbA1-PSII and PsbA3-PSII as those related to the sulfoquinovosyldiacylglycerol, the water molecules and the H-bond network.