Lipid-Mediated Modulation of mGluR2 Embedded in Micelle and Bilayer Environments: Insights from Molecular Dynamics
Lipid-Mediated Modulation of mGluR2 Embedded in Micelle and Bilayer Environments: Insights from Molecular Dynamics
Khodadadi, E.; Badiee, S. A.; Khodadadi, E.; Moradi, M.
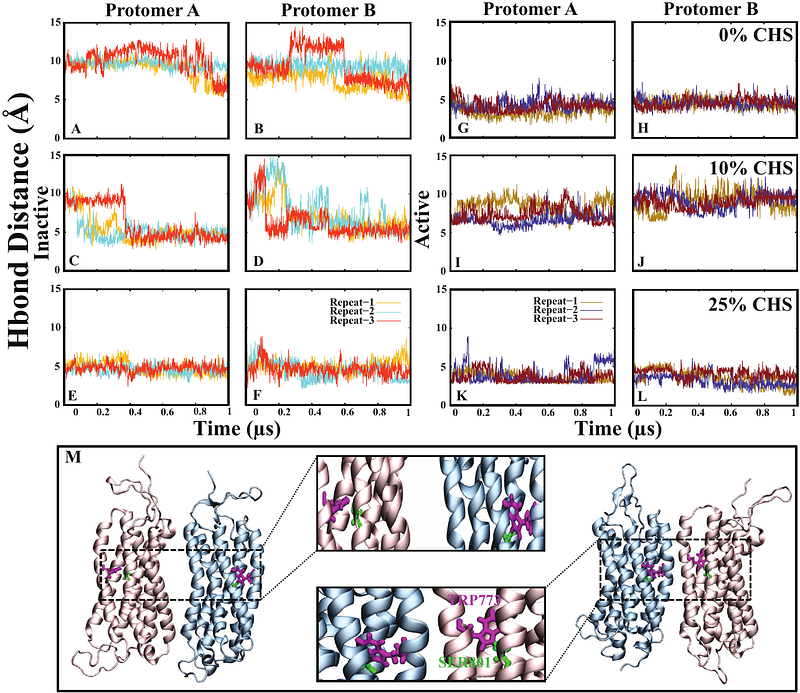
AbstractMetabotropic glutamate receptor 2 (mGluR2), a subclass C member of the G protein-coupled receptor (GPCR) superfamily, is essential for regulating neurotransmitter signaling and facilitating synaptic adaptability in the central nervous system. This receptor, like other GPCRs, is highly sensitive to its surrounding lipid environment, where specific lipid compositions can influence its stability, conformational dynamics, and function. In particular, cholesteryl hemisuccinate (CHS) plays a critical role in stabilizing mGluR2 and modulating its structural states within cellular membranes and micellar environments. However, the molecular basis for this lipid-mediated modulation remains largely unexplored. To investigate the effects of CHS and lipid composition on mGluR2, we employed all-atom molecular dynamics simulations of mGluR2 embedded in both detergent micelles (BLMNG and CHS) and a POPC lipid bilayer containing 0 %, 10 %, and 25 % CHS. These simulations were conducted for both active and inactive states of the receptor. Our findings reveal that CHS concentration modulates mGluR2 structural stability and conformational behavior, with a marked impact observed within transmembrane helices TM1, TM2, and TM3, which constitute the core of the receptor transmembrane domain. In micelle environments, mGluR2 displayed unique conformational dynamics influenced by CHS, underscoring the receptor sensitivity to its lipid surroundings. Notably, a CHS concentration of 10 % elicited more pronounced conformational changes than either cholesterol-depleted (0 %) or cholesterol-enriched (25 %) systems, indicating an optimal CHS range for maintaining structural stability. Our study provides atomistic insights into how lipid composition and CHS concentration impact mGluR2 conformational landscape in distinct micelle and bilayer environments. These findings advance our understanding of lipid-mediated modulation in GPCR function, highlighting potential avenues for receptor-targeted drug design, particularly in cases where lipid interactions play a significant role in therapeutic efficacy.