Large-scale bidirectional arrayed genetic screens identify OXR1 and EMC4 as modifiers of α-synuclein aggregation
Large-scale bidirectional arrayed genetic screens identify OXR1 and EMC4 as modifiers of α-synuclein aggregation
Neupane, S.; Nikolic, L.; Maraio, L.; Goiran, T.; Karpilovsky, N.; Sellitto, S.; Bouris, V.; Yin, J.-A.; Melki, R.; Fon, E. A.; De Cecco, E.; Aguzzi, A.
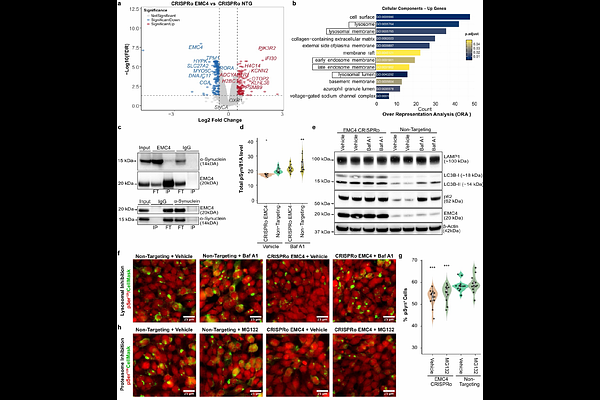
AbstractIn Parkinson\'s disease and other synucleinopathies, -synuclein (-Syn) misfolds and forms Ser129-phosphorylated aggregates (pSyn129). The factors controlling this process are largely unknown. Here, we used arrayed CRISPR-mediated gene activation and ablation to discover new pSyn129 modulators. Using quadruple-guide RNAs (qgRNAs) and Cas9, or an inactive Cas9 version fused to a synthetic transactivator, we ablated 2304 and activated 2428 human genes related to mitochondrial, trafficking and motility function in HEK293 cells. After exposure of cells to -Syn fibrils, pSyn129 signals were recorded by high-throughput fluorescent microscopy and aggregates were identified by image analysis. We found that pSyn129 was increased by activating the mitochondrial protein OXR1, which decreased ATP levels and altered the mitochondrial membrane potential. Instead, pSyn129 was reduced by ablation of the endoplasmic reticulum (ER)-associated protein EMC4, which enhanced ER-driven autophagic flux and lysosomal clearance. OXR1 activation preferentially modulated cellular reactions to fibrils derived from multiple system atrophy (MSA) patients, whereas EMC4 ablation broadly reduced pSyn129 across diverse -Syn polymorphs. These findings were confirmed in human iPSC-derived cortical and dopaminergic neurons, where OXR1 preferentially promoted somatic aggregation and EMC4 reduced both somatic and neuritic aggregates. These results uncover previously unrecognized roles for OXR1 and EMC4 in -Syn aggregation, thereby broadening our mechanistic understanding of synucleinopathies.