Unexpected Molecular Mechanism of Orc6-Based Meier-Gorlin Syndrome: Insights from a Humanized Drosophila Model
Unexpected Molecular Mechanism of Orc6-Based Meier-Gorlin Syndrome: Insights from a Humanized Drosophila Model
Balasov, M.; Akhmetova, K.; Chesnokov, I.
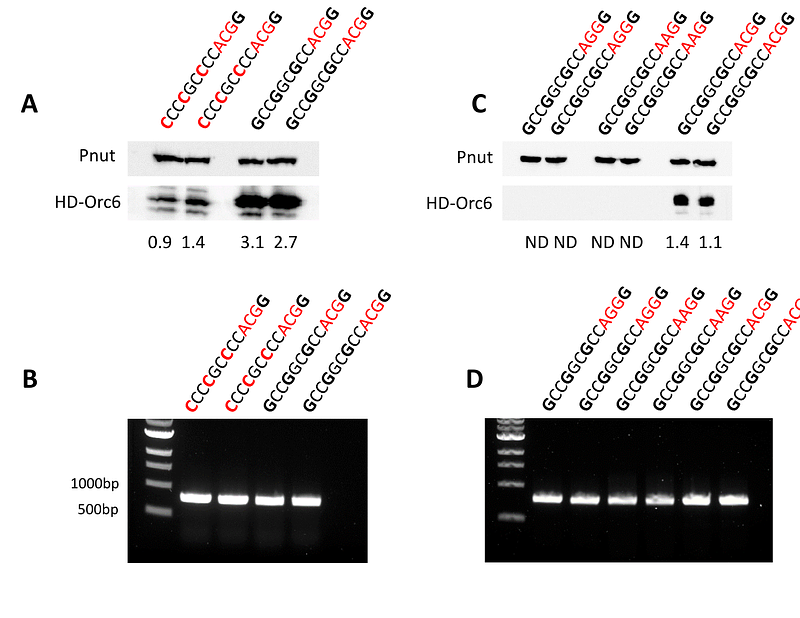
AbstractMeier-Gorlin syndrome (MGS) is a rare autosomal recessive disorder characterized by microtia, primordial dwarfism, and skeletal abnormalities. Patients with MGS often carry mutations in genes encoding the subunits of the Origin Recognition Complex (ORC), components of the pre-replicative complex and replication machinery. ORC6, an essential ORC subunit, plays a critical role in both DNA replication and cytokinesis. Approximately 30% of reported ORC6-related MGS cases exhibit compound heterozygosity for the ORC6 variants c.2T>C (p.Met1Thr) and c.449+5G>A. The c.2T>C mutation disrupts the start ATG codon by changing it to ACG, potentially initiating translation at an alternative downstream in frame Methionine (Met20), while c.449+5G>A results in in-frame exon skipping. Both mutations are predicted to produce significantly truncated ORC6 proteins with impaired functionality. In this study, using humanized ORC6 based Drosophila model, we demonstrated that these truncated proteins fail to rescue orc6 deletion. Instead, our findings reveal that the strong Kozak sequence, naturally present in human ORC6 mRNA, promotes translation from a non-canonical ACG codon. Rescued flies demonstrated a phenotype that we observed earlier for other MGS mutants in Drosophila. These results provide compelling evidence that MGS patients with c.2T>C/c.449+5G>A mutation rely on full size ORC6 protein initiated from a non-canonical ACG start codon.