A stress-NRF2 response axis polarises tumor macrophages and undermines immunotherapy
A stress-NRF2 response axis polarises tumor macrophages and undermines immunotherapy
Schaer, D. J.; Schulthess-Lutz, N.; Baselgia, L.; Kunasingam, K.; Humar, R.; Hansen, K.; Vallelian, F.
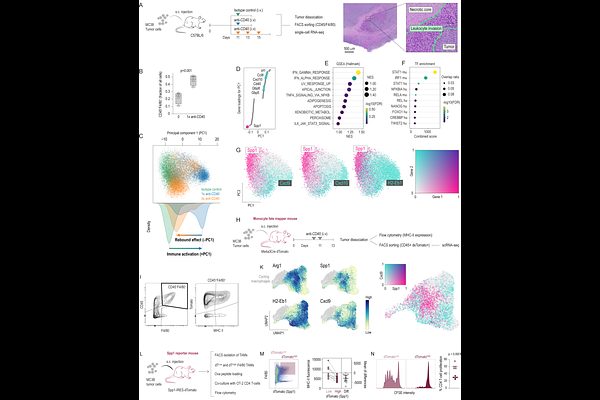
AbstractTumor-associated macrophages (TAMs) can switch between immune-activating and cancer-promoting states; yet, the stress pathways that lock them into pro-cancerous states remain obscure. In MC38 colon tumors, repeated anti-CD40 or radiotherapy created necrosis that split TAMs into peripheral Cxcl9+ and peri-necrotic Spp1+ subsets. Spatial transcriptomics, single-cell RNA-seq, and Keap1-deficient mice showed that the latter are NRF2high "stress-TAMs", with immunosuppressive and tumor-promoting activity. The same NRF2 activation gradient separates pro-inflammatory CXCL9+ and anti-inflammatory SPP1+ TAMs across diverse human cancers. NRF2high TAMs silence IFN-STAT1 programmes, lose MHC-II and chemokine expression, fail to expand T cells, drive tumor cell invasion in 3D co-cultures, and foster metastasis. Constitutive hematopoietic NRF2 activation accelerated the growth of therapy-naive MMTV-PyMT breast tumors and markedly impaired anti-CD40 efficacy in MC38 subcutaneous and lung-metastasis models. Conversely, macrophage-specific Nrf2 deletion restored immunogenic TAMs and potentiated anti-CD40 and anti-PD-1 treatments. Thus, NRF2 constitutes a stress-response axis that fixes TAMs in a pro-cancer, therapy-resistant state; inhibiting it could revive the macrophage-T-cell amplification loop and broaden immunotherapy responses.