LMO2 regulates epithelial-mesenchymal plasticity of mammary epithelial cells
LMO2 regulates epithelial-mesenchymal plasticity of mammary epithelial cells
Sikandar, S. S.; Haro-Acosta, V.; Juarez, M. A.; Olander, A.; Fetter, I. J.
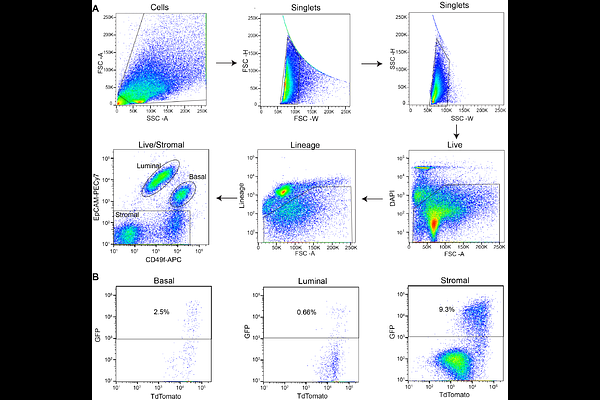
AbstractCellular plasticity in mammary epithelial cells enables dynamic cell state changes essential for normal development but can be hijacked by breast cancer cells to drive tumor progression. However, the molecular factors that maintain cellular plasticity through the regulation of a hybrid cell state (epithelial/mesenchymal) are not fully defined. As LMO2 has been previously shown to regulate metastasis, here we determined the role of LMO2 in the normal mammary epithelial cells. Using lineage tracing and knockout mouse models we find that Lmo2 lineage-traced cells persist long-term in the mammary gland, both in the luminal and basal layer but have limited proliferative potential. Lmo2 loss does not impact mammary gland development, but acute deletion decreases in vivo reconstitution. Moreover, LMO2 knockdown in mouse and human mammary epithelial cells (MECs) reduces organoid formation. We find that LMO2 maintains a hybrid cell state in MECs and LMO2 knockdown promotes mesenchymal differentiation. Transcriptional profiling of LMO2 knockdown cells reveals significant enrichment in the epithelial-mesenchymal transition (EMT) pathway and upregulation of MCAM, a negative regulator of regenerative capacity in the mammary gland. Altogether, we show that LMO2 plays a role in maintaining cellular plasticity in MECs, adding insight into the normal differentiation programs hijacked by cancer cells to drive tumor progression.