Identification of two novel DnaJ chaperone family proteins as modifiers of Huntingtin aggregates
Identification of two novel DnaJ chaperone family proteins as modifiers of Huntingtin aggregates
Deo, A.; Ghosh, R.; Ahire, S.; Majumdar, A.; Bose, T.
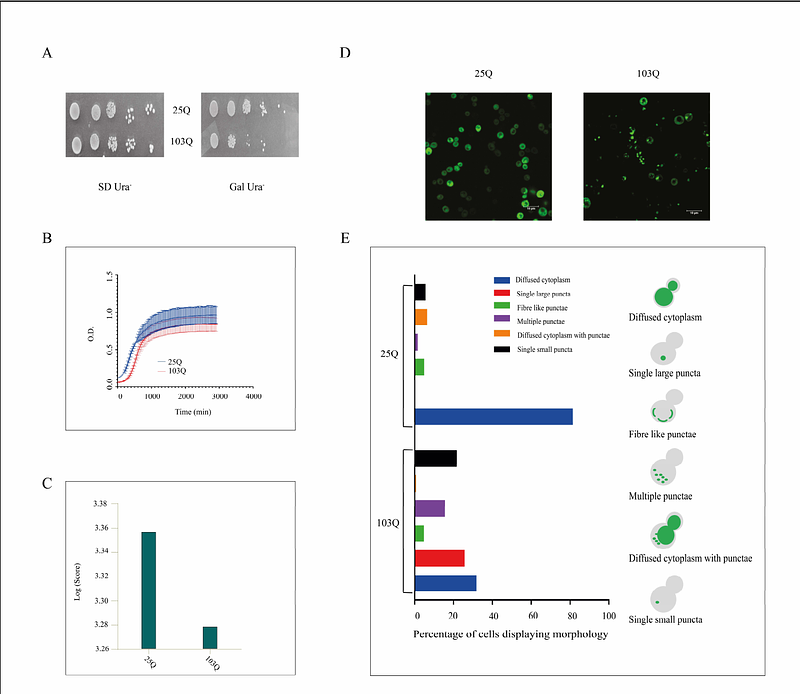
AbstractHuntington\'s disease (HD) is a rare neurodegenerative disease. It is caused due to aggregation of Huntingtin (HTT) protein containing Q repeats more than 40. Similar protein aggregation is also a hallmark of several other neurodegenerative diseases related to loss of cognitive function. In search of modifiers of HTT aggregation, we have screened putative chaperone proteins from Drosophila in both fly and yeast model of HD. DnaJ chaperones were screened by evaluating HTT protein aggregation related phenotypes using growth assays for studying the growth rate of the cells, imaging studies, and gel-based approaches like semi-denaturing detergent agarose gel electrophoresis (SDD-AGE). Our screening led us to categorize several proteins as suppressors and enhancers of the HTT associated phenotypes. Out of the 40 chaperones and co-chaperones, two chaperones that came up strikingly were CG5001 and P58IPK. Protein aggregation was found to be reduced in both S2 cells and Drosophila transgenic lines with HTT103Q in presence of these class of chaperones. As these DnaJ chaperones have protein sequence similarity across species, these might be used as possible tools to combat the effects of neurodegenerative diseases, as evidenced specifically in Huntington\'s disease and Amyotrophic Lateral Sclerosis (ALS).