Alpha-synuclein aggregation and dopaminergic neuron death in a new mouse model of Parkinson disease expressing human full-length and C-terminally truncated 1-120 alpha-synuclein.
Alpha-synuclein aggregation and dopaminergic neuron death in a new mouse model of Parkinson disease expressing human full-length and C-terminally truncated 1-120 alpha-synuclein.
Longhena, F.; Zheng, Z.; Faustini, G.; Wegrzynowicz, M.; Carlson, E.; Casadei, N.; Riess, O.; Bellucci, A.; Spillantini, M. G.
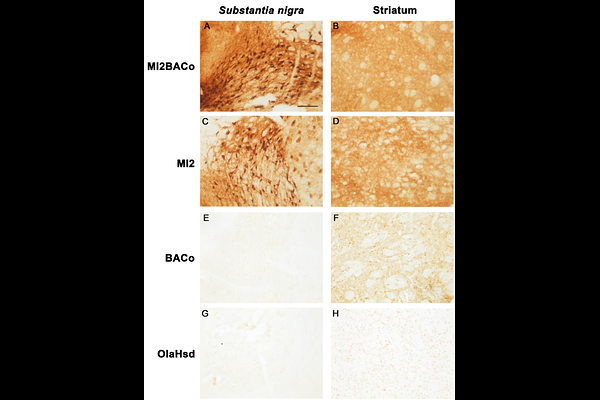
AbstractParkinson disease (PD) is neuropathologically characterized by the presence of Lewy bodies and Lewy neurites made of aggregated alpha-synuclein (aSyn). Several studies have shown that Lewy bodies contain C-terminally truncated aSyn which is more prone to fibrillation and enhances the aggregation of human full-length aSyn in vitro. Nevertheless, in vivo studies addressing whether and how human C-terminally truncated aSyn may exacerbate the pathological aggregation and toxicity of human full-length aSyn are currently lacking. In the present study we aimed to determine whether the co-expression of human full-length and 1-120 truncated aSyn would enhance human full-length aSyn aggregation and toxicity. To this end we generated and characterized two novel alpha-synuclein mouse models. Specifically, we first produced a mouse line expressing full-length human aSyn under its own promoter (BACo mice) in the absence of endogenous mouse aSyn. Then, the BACo mice were crossed with the previously described MI2 mice, which express human 1-120 truncated aSyn under the control of the tyrosine hydroxylase (TH) promoter on a mouse aSyn null backgroundin order to obtain the MI2BACo mice, which express both human 1-120 truncated and full-length aSyn in a mouse aSyn null background. We found that aSyn aggregation was present and increased with age in both mouse lines but was significantly higher in the MI2BACo mice. These mice exhibited progressive nigrostriatal accumulation of aSyn aggregates which were often composed of full-length and 1-120haSyn, the latter being specifically recognized by using a novel antibody raised against the 1-120haSyn. In addition, we observed that the presence of both truncated and full-length aSyn in MI2BACo mice led to a faster and more pronounced aSyn aggregation which associated with more severe dopaminergic striatal fiber degeneration and nigral neuron loss. Collectively, our results indicate that the expression of human C-terminally truncated aSyn exacerbates human full-length aSyn pathology and support that the novel MI2BACo transgenic mouse line represents an innovative experimental model to study the biological basis of PD and test novel therapeutic approaches.