Cnot3 is required for male germ cell development and spermatogonial stem cell maintenance
Cnot3 is required for male germ cell development and spermatogonial stem cell maintenance
Chen, Q.; Malki, S.; Xu, X.; Bennett, B.; Lackford, B. L.; Kirsanov, O.; Geyer, C. B.; Hu, G.
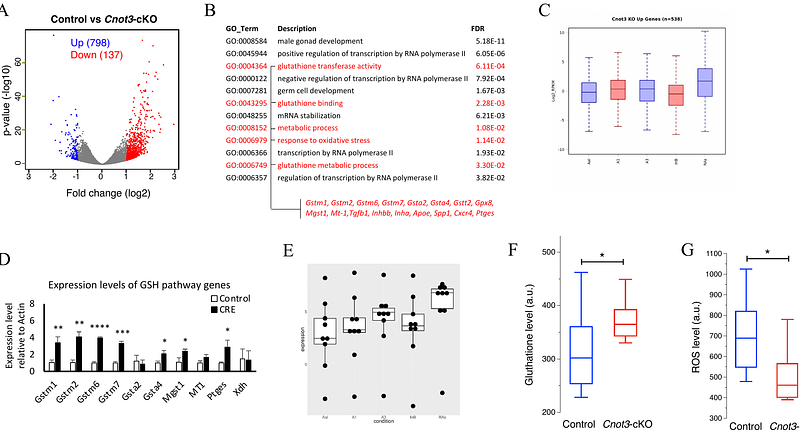
AbstractThe foundation of spermatogenesis and lifelong fertility is provided by spermatogonial stem cells (SSCs). SSCs divide asymmetrically to either replenish their numbers (self-renewal) or produce undifferentiated progenitors that proliferate before committing to differentiation. However, regulatory mechanisms governing SSC maintenance are poorly understood. Here, we show that the CCR4NOT mRNA deadenylase complex subunit CNOT3 plays a critical role in maintaining spermatogonial populations in mice. Cnot3 is highly expressed in undifferentiated spermatogonia, and its deletion in spermatogonia resulted in germ cell loss and infertility. Single cell analyses revealed that Cnot3 deletion led to the de-repression of transcripts encoding factors involved in spermatogonial differentiation, including those in the glutathione redox pathway that are critical for SSC maintenance. Together, our study reveals that CNOT3 (likely via the CCR4NOT complex) actively degrades transcripts encoding differentiation factors to sustain the spermatogonial pool and ensure the progression of spermatogenesis, highlighting the importance of CCR4NOT-mediated post-transcriptional gene regulation during male germ cell development.