Transcriptional Condensates Encode a "Golden Mean" to Optimize Enhancer-Promoter Communication across Genomic Distances
Transcriptional Condensates Encode a "Golden Mean" to Optimize Enhancer-Promoter Communication across Genomic Distances
Zhu, T.; Li, C.; Chu, X.
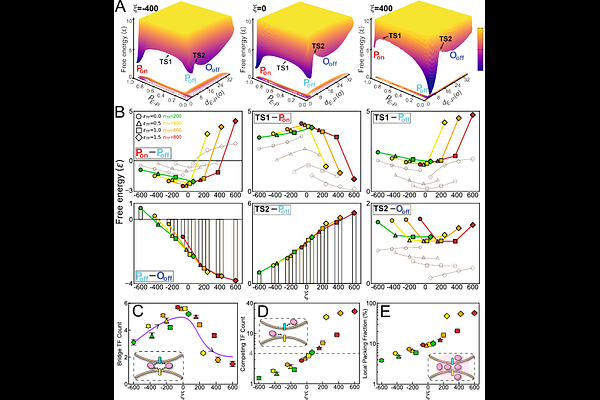
AbstractEnhancers regulate gene expression by physically contacting their target promoters, yet these contacts often span large genomic distances. Phase-separated condensates (droplet-like clusters) of transcription factors (TFs) are thought to facilitate such long-range enhancer-promoter (E-P) communication, but the quantitative principles underlying this mechanism remain unclear. Here we use polymer-based chromatin simulations to systematically vary the strength of TF clustering and the E-P genomic distance, examining their combined effects on E-P contact formation. We find that E-P contact frequency shows a non-monotonic dependence on the degree of TF clustering: contact frequency peaks at an intermediate TF abundance and TF-TF affinity, leading to a \"golden mean\" optimum. Two distinct regimes emerge: under weak TF-TF attraction, contact probability increases with condensate size, whereas strong TF attraction produces a peaked response that declines at high condensation levels. These results indicate that TF condensate acts as a tunable \"rheostat\", buffering E-P interactions against increasing genomic distances. However, excessive TF clustering leads to molecular crowding and competition that ultimately impair E-P communication. Our study, consistent with recent experiments, establishes a mechanistic framework at molecular level, where balanced TF condensation enables robust long-range E-P communication, reconciling the stochastic nature of chromatin dynamics with the fidelity of gene regulation.