WTAP is Transcriptionally Regulated by p65 and Promotes Inflammation through m6A Modification and Phase Separation
WTAP is Transcriptionally Regulated by p65 and Promotes Inflammation through m6A Modification and Phase Separation
Ge, Y.; Chen, R.; Ling, T.; Liu, B.; Huang, J.; Cheng, Y.; Lin, Y.; Chen, H.; Xie, X.; Xia, G.; Luo, G.; Yuan, S.; Xu, A.
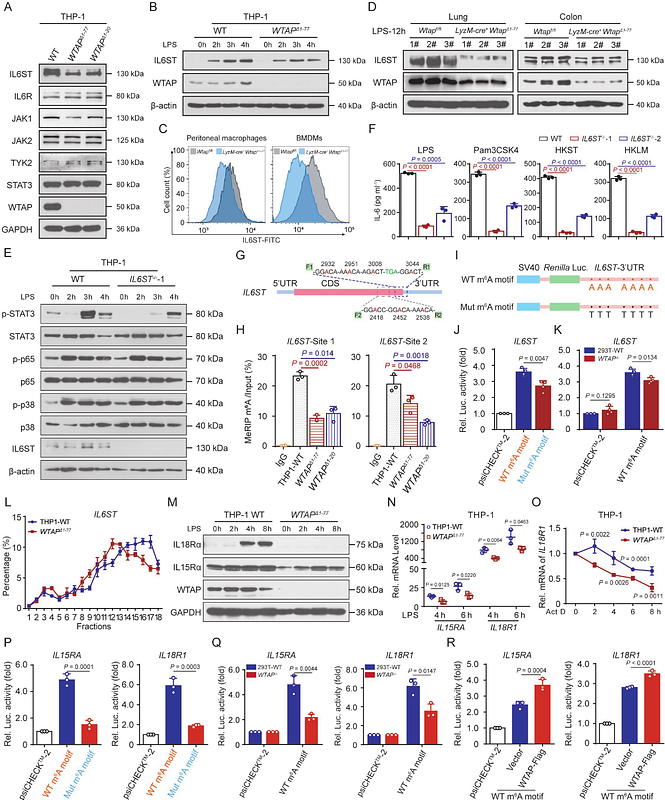
AbstractEmerging evidence has linked dysregulation of N6-methyladenosine (m6A) to inflammation and inflammatory diseases, but the underlying mechanism remains lacking. Here, we found that high m6A modification in a variety of hyperinflammatory states is p65-dependent, because WTAP, a key component of the writer complex, is transcriptionally regulated by p65 and its overexpression can lead to higher m6A modification. Mechanistically, upregulated WTAP is more prone to phase separation to facilitate the aggregation of \"writer\" complex to nuclear speckles and the deposition of m6A marks onto transcriptionally active inflammatory transcripts, thereby accelerating proinflammatory response. Furthermore, myeloid deficiency of WTAP attenuates the severity of LPS-induced sepsis and DSS-induced IBD. Thus, the proinflammatory effect of WTAP is a general risk-increasing mechanism, and interrupting the assembly of m6A writer complex by targeting the phase separation of WTAP to reduce the global m6A level may be a potential and promising therapeutic strategy for alleviating hyperinflammation.