Wnt11 Positively Regulates Neonatal Cardiomyocyte Maturation at the Interphase of Life via Frizzled 4 Receptor
Wnt11 Positively Regulates Neonatal Cardiomyocyte Maturation at the Interphase of Life via Frizzled 4 Receptor
Kang, X.; Moci, J.; Wolf, C.; Touma, M.
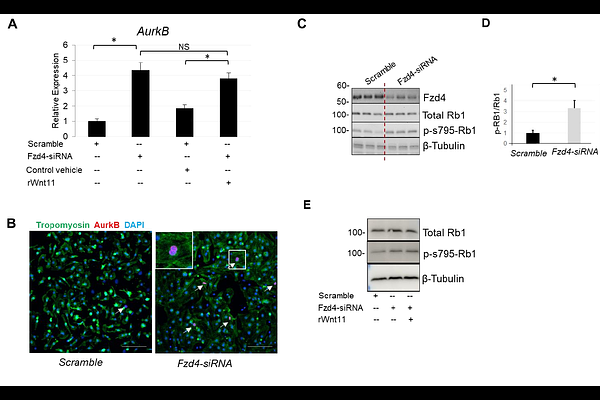
AbstractCongenital heart defects (CHDs) affect 1% of live births and remain the leading cause of infant morbidity and early mortality. While most studies focus on the genetic basis of CHDs, relatively little is known about the interplay between intrinsic signaling and external environmental factors in the progression of CHDs after birth during the perinatal circulatory transition window when environmental stress factors are prevalent. We recently explored such interplay through a newly identified gene-environment regulatory circuit involving Wnt11 signaling and systemic hypoxia. Specifically, we demonstrated that activation of the Wnt11/Rb1 axis is critical for normal chamber-specific development after birth. This regulatory switch is disrupted by systemic hypoxia more robustly in the right ventricle (RV) than the left ventricle (LV), leading to enhanced neonatal cardiomyocyte cell cycle activity in an RV-specific manner, resulting in delayed maturation and attenuation of ventricular patterning in response to systemic hypoxia stress in the neonatal heart. Furthermore, we found that the Wnt11/Rb1 axis is also inactivated in infantile hearts with cyanotic CHDs, such as tetralogy of Fallot (TOF), potentially contributing to hypoxia-associated RV abnormalities in this context. However, the molecular players of this signaling cascade in neonatal cardiomyocyte remain largely unknown. Herein, we report that Frizzled 4 (Fzd4) acts as a specific upstream receptor for Wnt11 in neonatal cardiomyocytes. Specifically, Fzd4 exhibited an expression pattern like Wnt11 in neonatal heart perinatal circulatory transition under normal and hypoxemic environments. Furthermore, Fzd4 loss in neonatal cardiomyocytes stimulated cardiomyocyte cell cycle activity and disrupted the Wnt11-Rb1 signaling axis mirroring the impact of the Wnt11-deficient cardiomyocyte phenotype. Finally, co-immunoprecipitation analysis confirmed the Wnt11-Fzd4 binding in isolated neonatal cardiomyocytes and intact hearts. These results demonstrate that Fzd4 is a specific and required upstream receptor for the Wnt11-Rb1 signaling activity in the neonatal heart and provides mechanistic insights into the essential role of Wnt11 as a key positive regulator of neonatal cardiomyocyte transition from proliferative to mature phenotype at the interphase of life.