Optogenetic control of mechanotransduction based on light-induced homodimerization of talin
Optogenetic control of mechanotransduction based on light-induced homodimerization of talin
Nishimura, R.; Barnett, S. F. H.; Jain, K.; Huang, Z.; Goult, B. T.; Kanchanawong, P.
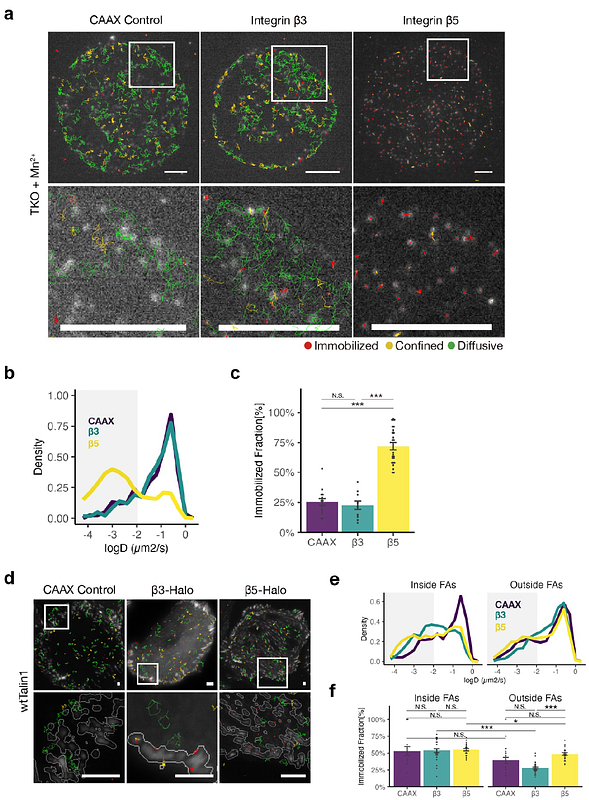
AbstractIntegrin-based cell adhesions (IACs) serve as primary sites where piconewton-scale actomyosin-generated mechanical forces are transmitted to the extracellular matrix (ECM), generating traction forces that drive cell-ECM responses including adhesion, migration, and mechano-signaling. Talin, a large (270 kDa) cytosolic adaptor protein, is the principal force-transmission protein in integrin-based adhesions, containing multiple mechanosensitive domains and protein-protein interaction sites that orchestrate molecular events in mechanosensing. As a highly modular multi-domain protein, talin has been identified as an effective target for chemogenetic and optogenetic manipulation of integrin-based mechanotransduction. However, a key limitation of previous approaches is the reliance on heterodimerization modules to control talin function, requiring the expression of two modified talin fragments. In practice, achieving precise expression levels in such a 2-component approach can be challenging, particularly when combined with other genetic tools. Since talin naturally contains a C-terminal dimerization domain that forms part of its actin-binding site, we reasoned that the molecularly engineered talin with a C-terminal optically-controlled homodimerizer could enable single-component optogenetic control of mechanotransduction. This approach would facilitate multiplexing with other molecular perturbations or experimental techniques. Here, we describe an opto-homodimerizable talin based on the pdDronpa1.2 optogenetic module, which enables optogenetic control of talin by a single construct. We demonstrate that light-induced talin dimerization promotes talin recruitment to IACs, adhesion formation, actin retrograde flow engagement, and downstream mechanotransduction signaling. Conversely, light-induced talin monomerization rapidly disassembles focal adhesions, disrupts talin-actin linkages, and accelerates actin retrograde flow, underscoring the critical roles of talin dimerization. Furthermore, our single-construct design allows facile multiplexing of optogenetic modulation of integrin-mediated mechanotransduction with super-resolution single-molecule tracking, revealing the essential role of talin dimerization for integrin v{beta}5 engagement.