Echinococcus granulosus antigen B acts as an LPS-scavenging lipoprotein in vitro preventing TLR4-mediated activation of dendritic cells
Echinococcus granulosus antigen B acts as an LPS-scavenging lipoprotein in vitro preventing TLR4-mediated activation of dendritic cells
Lagos Magallanes, S.; Beasley Lomazzi, A.; Zamarreno, F.; Carrion, F.; Flo, M.; Dutto, J.; Julve, J.; Costabel, M.; Maccioni, M.; Folle Lopez, A. M.; Ferreira Vazquez, A. M.
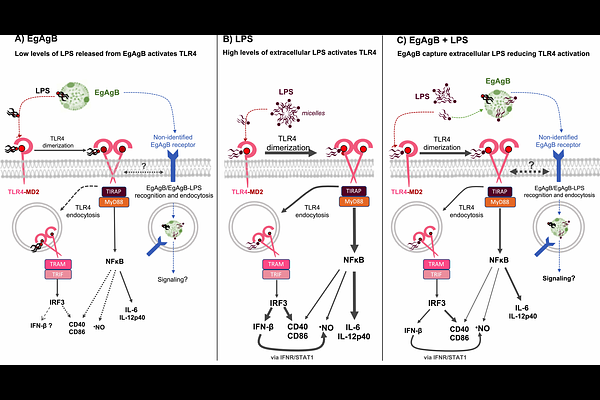
AbstractEchinococcus granulosus sensu lato antigen B (EgAgB) is a major parasite lipoprotein, produced by the hydatid and released at the host-parasite interface. Accumulating evidence supports that EgAgB may exert immunomodulatory effects on myeloid cells; however, the underlying molecular mechanisms remain poorly understood. We examined the impact of native EgAgB (nEgAgB) and recombinant EgAgB8/1 (rEgAgB) on lipopolysaccharide (LPS)-induced activation of bone marrow-derived dendritic cells (BMDC), to help elucidate these mechanisms. Both immunoaffinity-purified nEgAgB or rEgAgB induced modest BMDC activation, indicated by the production of IL-6, IL-12p40, and nitric oxide, but not IFN-{beta}. This activation was primarily attributed to LPS traces in EgAgB preparations since it was nearly abolished by a specific TLR4 inhibitor and in Tlr4-/- BMDC, while EgAgB binding to BMDC was TLR4-independent. Notably, both nEgAgB and rEgAgB inhibited LPS-induced cytokine and nitric oxide production, and disrupted TLR4 dimerization and endocytosis. Competitive binding assays showed that EgAgB and human high-density lipoprotein (hHDL) similarly inhibited LPS binding to macrophages and BMDC; however, EgAgB more effectively suppressed LPS-induced cytokine secretion. Contrastingly, EgAgB did not modulate BMDC responses to lipoteichoic acid, unlike hHDL. Using dynamic light scattering and an ELISA-like assay, we demonstrated a higher potential of EgAgB to bind LPS than hHDL. Additionally, docking analyses suggest the presence of a defined LPS-binding interface in EgAgB8/1 subunit. Overall, these findings reveal a novel binding property of EgAgB, which enables it to act as an extracellular LPS scavenger, interfering with TLR4-mediated LPS recognition and downstream proinflammatory responses in myeloid cells.