Single-cell RNA-seq reveals trans-sialidase-like superfamily gene expression heterogeneity in Trypanosoma cruzi populations
Single-cell RNA-seq reveals trans-sialidase-like superfamily gene expression heterogeneity in Trypanosoma cruzi populations
Inchausti, L.; Bilbao, L.; Campo, V. A.; Garat, J.; Sotelo-Silveira, J.; Rinaldi, G.; Howick, V. M.; Duhagon, M. A.; G. De Gaudenzi, J.; Smircich, P.
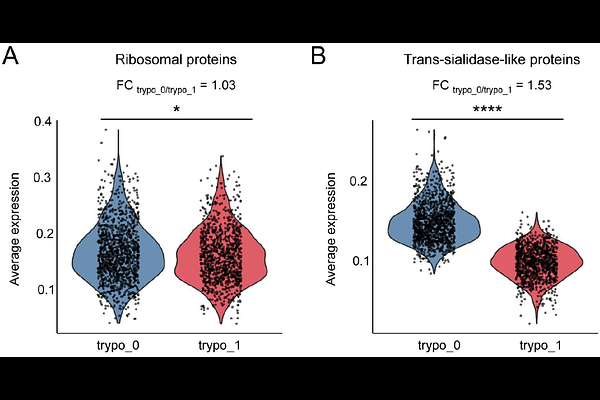
AbstractTrypanosoma cruzi, the causative agent of Chagas disease, presents a major public health challenge in Central and South America, affecting approximately 8 million people and placing millions more at risk. The T. cruzi life cycle includes transitions between epimastigote, metacyclic trypomastigote, amastigote, and blood trypomastigote stages, each marked by distinct morphological and molecular adaptations to different hosts and environments. Unlike other trypanosomatids, T. cruzi does not employ antigenic variation but instead relies on a diverse array of cell-surface-associated proteins encoded by large multi-copy gene families (multigene families), essential for infectivity and immune evasion. This study analyzes cell-specific transcriptomes using single-cell RNA sequencing of amastigote and trypomastigote cells to characterize stage-specific surface protein expression during mammalian infection. Through clustering and identification of cell-specific markers, we assigned cells to distinct parasite developmental forms. Analysis of individual cells revealed that surface protein-coding genes, especially members of the trans-sialidase TcS superfamily (TcS), are expressed with greater heterogeneity than single-copy genes. Additionally, no recurrent combinations of TcS genes were observed between individual cells in the population. Our findings thus reveal transcriptomic heterogeneity within trypomastigote populations where each cell displays unique TcS expression profiles. Focusing on the diversity of surface protein expression, this research aims to deepen our understanding of T. cruzi cellular biology and infection strategies.